11th Sep 2025 07:00
LEI: 549300Q7EXQQH6KF7Z84
11 September 2025
RTW Biotech Opportunities Ltd
Half-Yearly Report for the six months ended 30 June 2025
Following a resilient period of performance, the Company is well positioned to capture value from a diversified portfolio of otherwise inaccessible opportunities.
RTW Biotech Opportunities Ltd (the "Group", "RTW Bio" or the "Company"), the London Stock Exchange-listed investment company focused on identifying transformative assets with high growth potential across the life sciences sector, is pleased to announce its Half Year Results for the six months ended 30 June 2025.
Roderick Wong, MD, Managing Partner and Chief Investment Officer of RTW Investments, LP (the "Investment Manager") commented:
"The first half of 2025 saw one of the most volatile periods for the biotech sector since the Company's launch. Despite the headwinds which led to the NAV falling during the period, the Company continues to outperform its reference benchmarks and the wider peer group over the long term. Following period end, we were pleased to see that RTW Bio will be included in the FTSE All Share from 22 September.
"With new FDA leadership and the launch of the 'Make American Biotech Accelerate' initiative, the policy backdrop has turned increasingly supportive for innovation. We see these changes as a tailwind that could meaningfully accelerate the path from scientific breakthrough to patient benefit.
"While macro headlines around tariffs and drug pricing have stirred market volatility, the practical impact on most biotech companies remains limited. Tariff risks are muted given the sector's domestic focus, and the re-emergence of the Most Favoured Nation drug pricing concept is primarily a large pharma issue. In parallel, public market valuations remain at historically attractive levels, with nearly half of U.S. biotech companies trading below their cash balance. This dislocation - the fifth consecutive year of XBI underperformance versus the S&P 500 - in our view, represents a compelling opportunity for long term investors.
"Large pharma's looming patent cliffs are adding urgency to the M&A cycle, with $49 billion in announced deals for the first half already surpassing the full-year 2024 total. Cash-rich acquirors are actively seeking late-stage, high-impact assets, and the current valuation environment is fertile ground for strategic transactions. We expect deal activity to accelerate in the second half as political uncertainty subsides and the need for pipeline replenishment intensifies.
"In short, the biotech industry is navigating a complex but increasingly constructive environment. Policy momentum is leaning pro-innovation, valuations are at multi-year lows, and strategic buyers are stepping in with unprecedented balance sheet firepower. For investors able to look through the short-term noise, the combination of regulatory support, scientific progress, and strategic demand points to a potentially powerful re-rating as we look ahead to the second half and beyond."
Financial Highlights
| As at 30 June 2025 | Change over period | As at 31 December 2024 | As at 30 June 2024 |
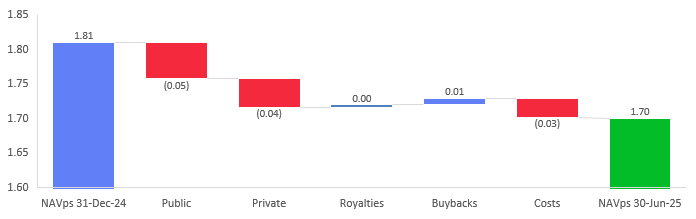
Net asset value (Ordinary Shares) | $561.0 million | (6.0%) | $606.9 million | $655.4 million |
Net asset value per Ordinary Share | $1.70 | (6.0%) | $1.81 | $1.95 |
Ordinary Share price | $1.21 | (13.6%) | $1.40 | $1.55 |
Share price discount to Net Asset Value | (28.8%) | (6.0%) | (22.8%) | (20.9%) |
Number of Ordinary Shares in issue | 330.1 million | (1.7%) | 335.7 million | 335.7 million |
Russell 2000 Biotech Index | (11.4%) | |||
Nasdaq Biotech Index | (1.9%) | |||
AIC Biotechnology & Healthcare Sector | (0.2%) |
Portfolio highlights
· | 5 new private companies added in the period: These included American Laboratories (1.4% of NAV) which specialises in the production of enzymes, proteins, and flavours used across several industries, and Prolium Bioscience (1.3% of NAV), a new RTW Investments created company focused on autoimmune diseases. |
· | 2 significant capital markets events: The IPO of obesity-focused Metsera, which has performed strongly since launch (+7.4% since IPO), bodes well for RTW Bio's investments in the space. The reverse merger in April of Jade Biosciences, merging with Aerovate Therapeutics, now trades on the NASDAQ under ticker "JBIO". |
· | Portfolio skewed towards later-stage companies: The portfolio's NAV exposure by development stage is 64% in clinical programs, 32% in commercial products, and 4% in pre-clinical programs. |
As of 30 June 2025, 70% of NAV was invested in public companies1 (2024: 65%), 32% was invested in private holdings (2024: 30%) and 3% of NAV was royalty exposure (2024: 3%).
1. In prior periods exposure was presented as fair market value +/- accruals as a percentage of NAV, summing to 100%. Beginning with this report, exposure will be shown as economic exposure as a percentage of NAV, the result of which could be more or less than 100%.
2. Development stage exposures apply to portfolio companies with 1% or greater exposure. Exposures have been converted to sum to 100%. In prior periods, development stage exposures were presented as the number of companies vs % of NAV, and on what was previously referred to as the "core" portfolio.
Public Investments
· | As at 30 June 2025, 70% of NAV was invested in public portfolio companies. The increase in exposure from 65% (as at 31 December 2024) reflects relative outperformance over the period versus private investments in addition to two privately-held positions going public. In January, Beta Bionics went public and raised gross proceeds of $204 million, trading under the ticker "BBNX". In April, Jade Biosciences completed a reverse merger with Aerovate Therapeutics and now trades on the NASDAQ under the ticker "JBIO". |
· | Akero was the strongest public contributor, with its share price rising 91.8% and adding 3.8% to NAV per Ordinary Share. The company reported positive clinical data for its lead asset, efruxifermin (EFX), in MASH, a progressive liver disease with limited treatment options. Shortly after, market speculation emerged around a potential strategic acquisition, further boosting investor sentiment. |
· | UroGen also performed well, contributing 1.5% to NAV. The company secured FDA approval for ZUSDURITM, a non-surgical treatment for a common form of bladder cancer, complementing its existing commercial product, Jelmyto®. This regulatory milestone helped drive a 28.6% increase in the share price over the period. |
· | Rocket was the largest public detractor, with its share price falling 80.5% and reducing NAV per Ordinary Share by -4.4%. During Rocket's pivotal Phase 2 trial for RP-A501 in Danon disease, a patient tragically died and the process was subject to a clinical hold. Rocket actively engaged with the FDA and the clinical hold was subsequently released in August. Phase 2 trials were restarted with a recalibrated dose and a revised immunomodulatory regimen. Rocket continues to advance a broader pipeline targeting rare genetic cardiomyopathies and haematologic disorders. |
· | Dyne shares fell in January after release of updated data from its Phase 1/2 ACHIEVE trial for DYNE-101, a treatment for myotonic dystrophy type 1 (DM1). While the data were positive, investors reacted negatively to the magnitude of the improvements. Additionally in June, Dyne's revised accelerated approval strategy for DM1 drove a delay in its regulatory timeline, also contributing to negative sentiment, and reducing NAV per Ordinary share during the period by -2.0%. |
Private Investments
· | As at 30 June 2025, private investments accounted for 32.4% of NAV across 36 investments. The increase in exposure from 30.3% (as at 31 December 2024) reflects five new investments over the period. These included American Laboratories (1.4% of NAV), which specialises in the production of enzymes, proteins, and flavours used across several industries, and Prolium Bioscience (1.3% of NAV), a new RTW company creation focused on autoimmune diseases. |
· | Among the Company's private investments, Corxel was the top contributor, adding 1.1% to the NAV per Ordinary Share. Corxel is an RTW Investments created company that is developing CX11, an oral, once-daily GLP-1 receptor agonist for obesity. In June, it enrolled the first patient in its U.S. Phase 2 trial, following positive results from a China Phase 2 trial conducted by partner Vincentage, where CX11 showed strong weight loss efficacy and a favourable safety profile. Topline data from the U.S. trial is expected in H1 2026. During the period, Corxel also received a dividend distribution linked to the sale of Aficamten to Sanofi. |
· | Artios was the largest detractor at -3.0%. In May, Artios announced encouraging Phase 1/2a data from its lead clinical oncology candidate, ART0380. Importantly, this data hits multiple indications, meaning that development costs (and therefore capital requirements) will likely be higher than expected. We remain positive on the promising clinical trial data from ART0380, which offers the potential to transform outcomes for individuals with hard-to-treat cancers, an area of high unmet need. |
· | Since admission, the Group has made 73 private investments. At 30 June 2025, 33 of these positions had experienced liquidity events (i.e., go-public or acquisition). The average holding period as private was 17 months and the average MOIC to the liquidity event was 1.7x. 23 of these positions have either concurrently or subsequently been exited in full at an average MOIC of 2.3x. |
Royalty Investments:
· | The Group's royalty positions, representing 2.8% of NAV, added a modest amount to the NAV per Ordinary Share. The underlying assets have performed well in the first half of 2025. Sales of Lumryz, marketed by Avadel Pharmaceuticals, have continued to perform well. The company raised its 2025 revenue guidance to $255-$265M, from $240-260M. |
· | The Jelmyto royalty asset, marketed by UroGen Pharma, also continues to perform well. UroGen maintained its 2025 sales growth guidance for Jelmyto at 8-12% growth. It reported long-term follow-up from the OLYMPUS trial that showed Jelmyto's median duration of response to be 47.8 months, adding positive long-term validation to Jelmyto's already strong clinical profile. On 12 June UroGen's UGN-102 asset received approval from the FDA and started marketing the drug as "Zusduri" from 1 July. The royalty contract with UroGen receives payments from the sale of both drugs. |
· | Looking forward, our royalties strategy has an exciting pipeline of opportunities including with Milestone Pharmaceuticals, centred on future royalty streams of its lead asset, Cardamyst, (a nasal spray medication to treat paroxysmal supraventricular tachycardia, PSVT). Funding of the royalty is contingent on FDA approval, which is expected in the second half of the year. |
Post Period-End Events
· | July performance rebounded with a NAV return of +7.8%. The Company has delivered an annualised NAV total return of +76.3% to end-July, outstripping the Russell 2000 Biotech Index, the Nasdaq Biotech Index, and the AIC Biotechnology and Healthcare Sector. |
· | On 3 September, FTSE Russell confirmed that RTW Bio's Ordinary Shares will be included in the FTSE All Share Index from market open on 22 September. |
· | Merck entered into a definitive agreement to acquire UK-based public portfolio company Verona Pharma Plc (NASDAQ: VRNA) for $107 cash per American Depositary Share, for a total transaction value of approximately $10 billion, at a 23% premium to Verona's closing price on 8 July, prior to the announcement. RTW Bio's position in Verona added 0.9% to the NAV per Ordinary Share return over July and the position has been sold. The transaction is subject to Verona shareholder and regulatory approvals and is expected to close in the fourth quarter of 2025. |
· | Private portfolio company Kailera Therapeutics and its partner Hengrui Pharma announced positive topline data from the China Phase 3 clinical trial of HRS9531 in individuals living with obesity or who are overweight. The trial met both primary endpoints, demonstrating superior weight loss and a greater percentage of participants achieving at least 5% body weight reduction compared to placebo. The safety profile was favourable and consistent with other GLP-1-based treatments, with most treatment-emergent adverse events being mild to moderate and gastrointestinal-related. |
· | Rocket Pharmaceuticals announced a strategic corporate reorganisation and pipeline prioritisation of its cardiovascular programs. It will implement a reduction in workforce of approximately 30%. Rocket anticipates that its existing cash resources will fund operations into 2027. It anticipates delays associated with the Fanconi Anemia (RP-L102) and Pyruvate Kinase Deficiency (RP-L301) programs. FDA approval of RP-L102 is no longer anticipated in 2026. |
· | After announcing after year end that it was halting work on its lead candidate after a failed Phase 2 study and the discontinuation of its entire pipeline, Cargo Therapeutics announced it had entered into a definitive agreement to be acquired by Concentra Biosciences. |
Company Updates
· | Historically, the Group's public investments have been split into "core public" and "other public" pools. Core public largely comprises of positions originally held privately and represents the primary driver of long-term performance, while other public originally served as a capital-efficient way to deploy excess cash (typically reflecting the exposures of the Investment Manager's private funds). As a matter of good corporate governance, we regularly garner feedback on how we can improve aspects of our delivery to shareholders. We understand that the distinction between core public and other public has become less meaningful to investors and separating the pools can obscure the fact that RTW Bio is predominantly publicly invested, particularly in a market where the valuation of private assets is under scrutiny. Going forward, listed holdings will be reported under a single public category, alongside private and royalties. This presentational change does not impact how the portfolio is constructed or managed. |
· | The Company repurchased 5,650,000 shares in the first half of 2025, representing 15% of all shares traded during the period. Together with the 8,500,000 shares bought back in 2024, these repurchases have been accretive to NAV per share and increased secondary market liquidity. Importantly, this also demonstrates the Board's strong conviction in the Investment Manager's capabilities, the portfolio valuation, and the exceptional opportunity to invest in innovative biotech today. |
Enquiries:
RTW Investments, LP - Investment Manager Oliver Kenyon Woody Stileman Krisha McCune (Investor Relations)
| +44 (0)20 7959 6362 |
Cadarn Capital - PR & IR Partner Lucy Clark (PR) David Harris (Distribution)
|
+44 (0)7984 184 461 / [email protected] +44 (0)7368 883 211 / [email protected] |
Deutsche Numis - Joint Corporate Broker Freddie Barnfield Nathan Brown
| +44 (0)20 7260 1000 |
BofA Securities - Joint Corporate Broker Edward Peel Alex Penney
| +44 (0)20 7628 1000 |
Altum (Guernsey) Limited Joanna Duquemin Nicolle Sadie Morrison
| +44 (0)1481 703 100 |
About Biotech Opportunities Ltd:
RTW Biotech Opportunities Ltd (LSE: RTW) is an investment fund focused on identifying transformative assets with high growth potential across the biopharmaceutical and medical technology sectors. Driven by a long-term approach to support innovative businesses, RTW Biotech Opportunities Ltd invests in companies developing next-generation therapies and technologies that can significantly improve patients' lives. RTW Biotech Opportunities Ltd is managed by RTW Investments, LP, a leading healthcare-focused entrepreneurial investment firm with deep scientific expertise and a strong track record of supporting companies developing life-changing therapies.
Visit the website at www.rtwfunds.com/rtw-biotech-opportunities-ltd for more information.
Highlights
30 June 2025 Financial Highlights
$561.0 million Ordinary NAV 31 Dec 2024: $606.9 million
| $1.70 NAV per Ordinary Share 31 Dec 2024: $1.81 |
+63.4% Ordinary NAV per share growth since inception³ 31 Dec 2024: +73.8%
|
+16.4% total shareholder return since admission 31 Dec 2024: +34.1% |
-6.0% Ordinary NAV per share growth YTD 31 Dec 2024: -4.6%
|
-13.3% total shareholder return YTD 31 Dec 2024: -0.6% |
1.3x Leverage² 31 Dec 2024: 1.2x
|
$1.21 price per Ordinary Share ¹ 31 Dec 2024: $1.40
|
1 The share price was $1.51 at 9 September 2025.
2 Leverage is calculated per the Commitment Method of the AIFMD. Real economic exposure at the fund and position level (1.0x) is ultimately what impacts NAV as some positions are partially or fully hedged.
3 The NAV per Ordinary Share at IPO was $1.04.
Portfolio Highlights in the period
Significant capital markets activities in the private portfolio: 1 IPO and 1 reverse merger
30 June 2024: 2 significant capital markets activities: 1 IPO, 1 reverse merger
| 5 new private companies added in the period
30 June 2024: 14 |
Private exposure¹: 32% Public exposure¹: 70% Royalty exposure¹: 3%
31 Dec 2024: Private 30%, Public 65%, Royalty 3%
| 32% NAV exposure to companies with commercial products²
4% NAV exposure to companies with pre-clinical programs²
64% NAV exposure to companies with clinical programs²
|
Average historical multiple on invested capital (MOIC) of private investments to liquidity event since inception: 1.7x 31 Dec 2024: 1.8x Average historical holding period of private investments to liquidity event: 17 months 31 Dec 2024: 14 months |
1 In prior periods exposure was presented as fair market value +/- accruals as a percentage of NAV, summing to 100%. Beginning with this report, exposure will be shown as economic exposure as a percentage of NAV, the result of which could be more or less than 100%.
2 Development stage exposures apply to portfolio companies with 1% or greater exposure. Exposures have been converted to sum to 100%. In prior periods, development stage exposures were presented as the number of companies vs % of NAV, and on what was previously referred to as the "core" portfolio.
RTW Bio's Purpose and Long-Term Strategy
Transforming the lives of millions
RTW Bio's long-term strategy is anchored in identifying sources of transformational innovations with significant commercial potential by engaging in deep scientific research and a rigorous idea generation process, which is complemented by years of investment, company building, and both transactional and legal expertise.
Why Invest?
RTW Bio offers shareholders the benefit of exposure to a wide swath of investments in private and public companies in a sector requiring specialist expertise that would typically be a barrier to entry for most. As a publicly traded investment company, RTW Bio democratises investing beyond the private fund universe. The following investment platforms (not a comprehensive list) provide direct access to buying shares:
· | AJ Bell |
· | Hargreaves Lansdown |
· | Interactive Investor |
· | Barclays Smart Investor |
· | Charles Stanley |
Chair's Statement
Dear Shareholders
On behalf of the Board, I am pleased to provide the Group's Interim Report and Accounts for the six-month period to 30 June 2025.
Performance
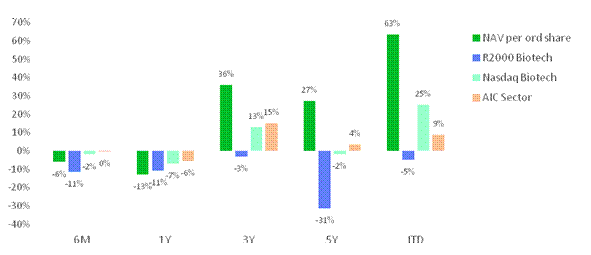
The first half of 2025 has been volatile for global markets and the biotech sector was not immune from this. The Group's NAV per Ordinary Share continues to outperform its reference indices and peer group over the long-term, although the NAV per Ordinary Share fell during the period. Over the six months to 30 June 2025, the Group delivered a NAV per Ordinary Share return of -6.0%, ahead of the Russell 2000 Biotechnology Index (-11.4%) and behind the Nasdaq Biotechnology Index (-1.9%). The Russell 2000 Biotechnology Index is heavily tilted towards smaller market cap companies, which aligns closely with RTW Bio's approach, while the Nasdaq Biotechnology Index provides broader exposure and includes larger companies.
Since IPO in October 2019, the Group's NAV per Ordinary Share has grown by 63.4%, materially ahead of the Russell 2000 Biotechnology Index (-4.8%), the Nasdaq Biotechnology Index (+25.2%), and the AIC Biotechnology & Healthcare Sector (+8.8%). The Group's performance is also ahead of both indices and the peer group over three-year (RTW Bio +35.9%, Russell 2000 Biotech -3.0%, Nasdaq Biotech +12.8%, AIC +3.6%) and five-year (RTW Bio +27.2%, Russell 2000 Biotech
-31.3%, Nasdaq Biotech -1.6%, AIC +3.6%) time periods.
Public Investments
Over the first half of the year, public investments contributed -2.9% to the NAV per Ordinary Share return. At the period end, 70.2% of the Group's portfolio was in public securities.
The largest contributor was Akero, where the share price rose by 91.8% over the period and delivered 3.8% to the NAV per Ordinary Share return. This followed promising data from its drug to reverse liver cirrhosis with Akero later rumoured to be exploring a potential sale. Rocket was the main detractor over the period, contributing -4.4% to the NAV per Ordinary Share return, after a young man treated with its gene therapy trial for Danon disease tragically died. Rocket actively engaged with the FDA and the clinical hold was subsequently released in August. Phase 2 trials were restarted with a recalibrated dose and a revised immunomodulatory regimen. We remain hopeful there is a path forward given the benefits seen in earlier patients.
Private Investments
The Company's private investments contributed -2.3% to the NAV per Ordinary Share return. At the period end, 32.4% of the Group's portfolio was in private assets.
Corxel announced major developments for its lead candidate, CX11, an oral GLP-1 receptor agonist for obesity and overweight: Corxel began enrolling patients in a U.S. Phase 2 trial following the release of positive results from a China Phase 2 trial, where CX11 demonstrated significant weight reduction across all doses. Corxel delivered a 1.1% contribution to performance over the period. Artios contributed -3.0% to returns. Encouraging clinical trial data from its lead oncology candidate hit multiple indications, meaning that development costs were forecast to be higher than expected. We remain positive on the company and its candidate, ART0380, which offers the potential to transform outcomes for individuals with hard-to-treat cancers, an area of high unmet need.
Royalties
Royalty financing, a growing non-dilutive capital source for life sciences companies launching first- or best-in-class therapies, involves an upfront payment in exchange for a percentage of future revenues, typically paid quarterly. RTW Bio accesses this model through its investment in 4010 Royalty Fund, managed by RTW Investments, which leverages its full life cycle platform to identify and structure deals with strong downside protection. RTW Bio's royalty exposure contributed modestly to NAV over the first half, with assets like Lumryz (Avadel) and Jelmyto (UroGen) performing well - both showing strong sales and regulatory progress. Looking ahead, 4010 has a promising pipeline with several term sheets in negotiation, supported by favourable market conditions for non-dilutive funding.
Discount and capital allocation
Discounts across the investment companies sector remain wide on a historical basis, driven by both structural and sentiment-related factors, particularly for the biotech space, which is impacted by ongoing political volatility. The Board recognises that the current share price discount to NAV remains wider than shareholders would wish and remains committed to taking steps to narrow it materially over time.
In response, the Company has actively repurchased 5,650,000 shares in the first half of 2025, representing 15% of all shares traded during the period. Together with the 8,500,000 shares bought back in 2024, these repurchases have been accretive to NAV per share and increased secondary market liquidity. Importantly, this also demonstrates the Board's strong conviction in the Investment Manager's capabilities, the portfolio valuation, and the exceptional opportunity to invest in innovative biotech today.
Buybacks are evaluated through a capital allocation lens, always anchored in our core objective: long-term capital growth. Given that our portfolio companies typically consume capital to advance through clinical development or early commercialisation, maintaining sufficient liquidity is critical to protect and grow value over time. This is especially relevant in today's market for small- and mid-cap biotech, where capital scarcity creates opportunity for those able to act swiftly. We continue to believe this is a once-in-a-generation moment to invest in public and private biotech.
Therefore, when allocating your capital, the Board carefully considers both the prevailing level of discount in the Company's shares and the opportunity to deploy capital at attractive valuations to deliver outsized long-term capital growth, whether by supporting existing investments or funding new opportunities. However, should the Group receive a significant cash inflow (e.g. through a portfolio position being acquired), it remains the Board's intention to consider the return of a portion of the realised gain to shareholders through buybacks.
Following period end, the Board was pleased to note the 3 September announcement from FTSE Russell that, following the quarterly review of the FTSE Russell UK Index Series and the resulting decision to allow the inclusion of non-GBP shares, RTW Bio's Ordinary Shares will be included in the FTSE All Share Index with effect from market open on 22 September. Inclusion in the FTSE All Share Index is likely to enhance our exposure to investors, develop greater UK and international awareness of RTW Bio and provide increased long-term access to capital. In due course, we expect index trackers and other passive strategies to initiate and build positions within RTW Bio, which should be positive for the Group's share price and its rating.
Simplification of portfolio breakdown
Historically, the Group's public investments have been split into "core public" and "other public" pools. Core public largely comprises positions originally held privately and represents the primary driver of long-term performance, while other public originally served as a capital-efficient way to deploy excess cash (typically reflecting RTW's private fund exposures).
As a matter of good corporate governance, we regularly garner feedback on how we can improve aspects of our delivery to shareholders. We understand that the distinction between core public and other public has become less meaningful to investors and separating the pools can obscure the fact that RTW Bio is predominantly publicly invested, particularly in a market where the valuation of private assets is under scrutiny. Going forward, listed holdings will be reported under a single public category, alongside private and royalties. This presentational change does not impact how the portfolio is constructed or managed.
Outlook
2025 began with renewed optimism for biotech, driven by a resurgence in M&A activity. The acquisition of Intra-Cellular Therapies by Johnson & Johnson in January served as a high-profile signal of strategic interest returning to the space, helping to lift sentiment and catalyse a rally in biotech indices. Innovation remained robust across key therapeutic areas, including obesity, oncology, and rare diseases, while the FDA maintained a strong pace of drug approvals. However, this early momentum was tempered by macroeconomic and political uncertainty around "Liberation Day". These factors contributed to persistent volatility, especially among small- and mid-cap biotech names.
Despite these headwinds, companies with differentiated pipelines and near-term catalysts have continued to attract capital, and the valuation reset of recent years created compelling entry points for long-term investors. The combination of scientific progress, strategic interest from large pharma, and a stabilising macro backdrop laid the foundation for a more constructive second quarter.
Looking ahead, the outlook for the remainder of 2025 is encouraging. Policy clarity is improving, with new FDA leadership reaffirming its commitment to innovation and regulatory flexibility. Initiatives such as "Make American Biotech Accelerate" (MABA) underscore the strategic importance of biotech to the U.S. economy. The risk of impact from tariffs is receding given the large on-shoring commitments made by big pharma and we are also seeing a potential resolution to the debate around drug pricing in the U.S. Further details on policy evolution is contained in the Investment Manager's report. Capital markets activity is beginning to reawaken, and a pipeline of upcoming clinical readouts and potential M&A catalysts is expected to drive renewed investor engagement. The sector is also benefiting from easing inflationary pressures and a more stable interest rate environment, which should support risk appetite and capital flows.
RTW Bio continues to provide investors with access to the most innovative and high-growth areas of the healthcare sector through a diversified portfolio spanning public, private, and royalty investments. Leveraging the access and strategic approach of its Investment Manager, RTW Investments, the Company's full life cycle strategy provides shareholders with exposure to often otherwise inaccessible opportunities and the opportunity to capitalise on emerging innovation, be that next-generation obesity therapies or the groundbreaking use of psychedelic medicine for patients with severe forms of depression. With a well-capitalised, liquid balance sheet and a high-conviction investment strategy, the Company is well-positioned to capture value as sentiment improves. The Board believes the second half of 2025 presents a compelling environment for biotech investing, defined by innovation, strategic momentum, and the potential for meaningful long-term returns.
William Simpson
Chair
10 September 2025
Report of the Investment Manager
Sector overview
The first half of 2025 has been marked by significant policy shifts and regulatory recalibrations in the U.S. healthcare landscape. RTW's Policy and Government Affairs team is actively engaging with policymakers on the administration's priorities, and we are thinking about political and regulatory risk within three buckets:
FDA
The appointment of Dr Marty Makary as FDA commissioner brought renewed support for regulatory flexibility and a proactive stance on accelerating innovation. Dr Vinay Prasad stepped down as head of CBER in July but rejoined the agency in August. His return as head of CBER at the FDA has not triggered volatility in rare disease or gene therapy stocks, suggesting that the market is now more familiar with his views and less reactive to leadership changes. This momentum extended to the Department of Health and Human Services, where RFK Jr launched the "Make American Biotech Accelerate" (MABA) initiative, stating in a social media post that 'We know the power of U.S. biotech. It's time to let it flourish…we're clearing the path to transform great science into real cures, at lower costs, and better health for the American people.' This is a signal of the sector's strategic importance to the U.S. and aligns with the National Security Commission on Emerging Biotechnology's push to position biotechnology as a national security priority through six pillars:
· | Pillar 1: Prioritize biotechnology at the national level |
· | Pillar 2: Mobilize the private sector to get U.S. products to scale |
· | Pillar 3: Maximize the benefits of biotechnology for defense |
· | Pillar 4: Out-innovate our strategic competitors |
· | Pillar 5: Build the biotechnology workforce of the future |
· | Pillar 6: Mobilize the collective strengths of our allies and partners |
We are already seeing signs of increased responsiveness in regulatory interactions and believe these reforms will accelerate the path to market for high-impact therapies. Overall, we believe that changes at the FDA may become a potential tailwind for biotech as the agency becomes more efficient and transparent while maintaining its pro innovation leaning.
Tariffs
President Trump announced "Liberation Day" on 2 April, when a raft of tariffs were imposed on imports into the U.S., although it is notable that pharma companies were initially excluded. After an initial sell-off, the market has recovered and the tariff risk for biotech appears to be largely behind us given the pharma sector's swift response. Over half of multinational pharmaceutical companies have already committed to 're-shore' nearly $300 billion in manufacturing in the US, including $55 billion by J&J, $50 billion by Roche, and $40 billion by Bristol Myers Squibb. We believe this swift industry response will likely soften the eventual impact of any manufacturing-related tariffs, potentially through exemptions or phased implementations.
Importantly, even in a worst-case scenario, we believe that the financial impact on biotech companies would be minimal. Unlike big pharma companies, biotech firms are less exposed to global supply chain dynamics as most are focused on or based in the U.S. and either out-licence or forgo ex-U.S. commercialisation altogether. With policy momentum shifting toward innovation and domestic investment, the tariff narrative has faded into the background, replaced by a more constructive dialogue around biotech as a national strategic asset.
Drug pricing
Most Favoured Nation (MFN) drug pricing is a policy concept aimed at aligning U.S. drug prices with those in lower-cost countries, particularly those in Europe. It re-emerged under the Trump administration as part of a broader effort to address what the White House views as "free riding" by foreign governments on American pharmaceutical innovation. The White House is considering various approaches to lower U.S. drug prices and we are cautiously optimistic that reasonable deals can get done. What's more, the Trump administration has made it clear that it is also interested in direct-to-consumer solutions to lower prices for U.S. patients, and we saw validation of that approach shortly after the period end on 17 July when Bristol Myers Squibb and Pfizer announced a "direct-to-patient" option for their blood thinning drug, Eliquis. We are also hearing progress on the European side that could mitigate MFN policies put in place in the U.S. In the worst-case scenario where no deal is reached, MFN is largely a pharma rather than a biotech problem, for similar reasons as outlined above with regard to tariffs.
Market overview
Public market valuations in biotech continued to be challenged in the first half, although with a notable divergence between the small-cap focused Russell 2000 Biotech Index (-11.4%) and the large-cap skewed Nasdaq Biotech Index (-1.9%). Given the policy uncertainty that has dominated the year so far, it is not surprising that the more defensive large-cap, commercial-stage names have outperformed.
Figure 1. Annual XBI vs S&P Performance
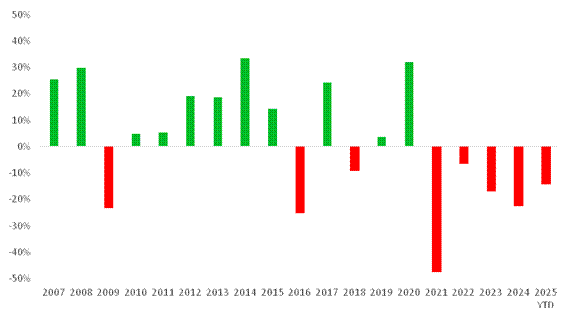
The first half of 2025 continues the underperformance of the XBI (S&P Biotech ETF) against the broader S&P 500. We are now in the fifth consecutive year of underperformance, which is remarkable as the XBI has never underperformed the S&P 500 for more than one consecutive year in the last 15 years. We believe this signals the compelling value potential of small- and mid-cap biotech. A parallel can be drawn with the flourishing biotech ecosystem in Hong Kong, where the Hang Seng Biotech Index fell 20% in 2024 yet rebounded 59% in the year to June and is up 115% for the year to date.
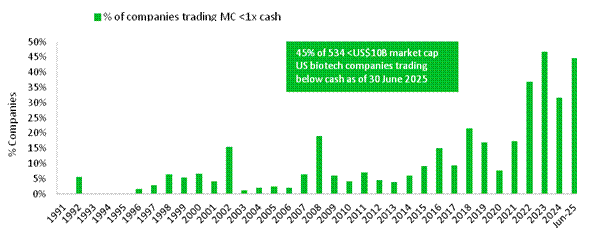
At 30 June, almost half of publicly-traded US biotech companies had market capitalisations lower than the level of cash on their balance sheets, an increase on the prior two years. The Nasdaq Biotech Index continues to trade at low EV/EBITDA and Price/Sales ratios. We believe that these levels represent an attractive point to put new money to work, particularly in late-stage biotech companies, and are favourable for M&A.
Figure 2: Percentage of US, small- and midcap biotech companies trading at less than cash on their balance sheets at 30 June 2025
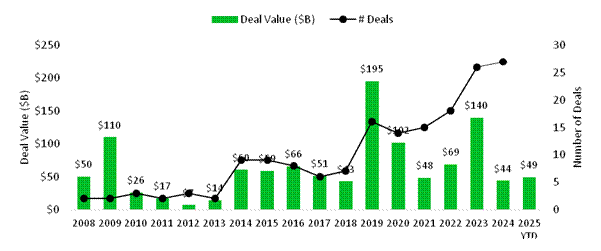
Patent cliffs and loss of exclusivity (LOE) remain a critical strategic concern for large pharma companies, with significant revenue at risk over the coming years as drugs 'go generic'. Ten blockbuster drugs (meaning those with >$1 billion sales per annum) face LOE issues in the near term and together these drugs generated a combined $164 billion in global sales in 2024. The most acute revenue erosion is expected in 2028, when multiple high-revenue products are set to lose exclusivity. This looming cliff is prompting large pharma companies to intensify their pipeline replenishment efforts, often through M&A and strategic collaborations; indeed, pharma and large-cap biotech have c. $1 trillion in 'dry powder' (cash and moderate debt capacity) to deploy in acquisitions. M&A accelerated in the first half of 2025, with $49 billion of deals announced, already ahead of the $44 billion seen in the whole of 2024. Marquee deals included J&J's $14.7 billion acquisition of Intra-Cellular Therapies in January, Sanofi's $9.9 billion acquisition of Blueprint Medicines in June, and Merck's $3.4 billion acquisition of SpringWorks Therapeutics in April. Strategic collaborations are also on the rise, such as the $11.1 billion deal between BioNTech and Bristol Myers Squibb announced in June. We expect M&A to accelerate in the second half of the year as volatility and political uncertainty subside, given the compelling valuations at which public biotechs trade and the imperative for large pharma to replace lost revenues.
Figure 3: US biotech M&A deal volumes and value
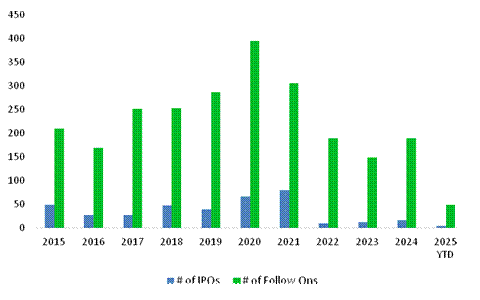
Biotech IPO activity remained subdued over the first half, constrained by persistent macroeconomic headwinds. Although a few high-quality, clinical-stage companies managed to go public, broader market conditions and mixed aftermarket performance of early 2025 IPOs continued to dampen broader issuance. Historically, the sector averaged around 50 IPOs annually from 2014-24, however, recent years have seen a sharp decline, with only 24 IPOs in 2024 and just 7 in 2025 so far. Notably, the 2025 IPO cohort has skewed toward later-stage companies (Phase 2 and beyond), a shift from the preclinical-heavy wave of 2020-21, reflecting investor preference for more de-risked assets in the current environment. We note the January IPO of obesity-focused Metsera, which has performed strongly since launch (+7.4%) which bodes well for RTW Bio's investments in the space. Private placement activity continued, with $10.4 billion raised across 148 deals, in-line with the $12.4 billion across 168 deals raised in the first half of 2024. The market remained bifurcated: while megarounds (>$100 million) accounted for over half of the capital raised, their frequency declined, and most were structured as tranched financings. Series A and B rounds saw robust step-ups, while Series C valuations remained volatile. Similar to the IPO market, investor appetite within private fundraises shifted towards later-stage opportunities.
The follow-on financing environment for biotech remained highly selective, with 37 secondary offerings raising approximately $7 billion, down sharply from 83 offerings and $18 billion in proceeds during the same period in 2024. Investor support was largely reserved for companies with clear value-driving catalysts, such as positive clinical data. Nonetheless, companies demonstrating fundamental success, particularly through clinical milestones, have continued to access capital markets effectively.
Figure 4: US Biopharma Financing Market - IPOs and Follow-Ons
In the first half of 2025, the FDA approved 16 new drugs, reflecting a measured but steady pace of regulatory activity. While the approval count is lower than in recent years, it includes several first-in-class therapies and drugs developed using novel modalities such as gene editing and RNA-based platforms. Our sentiment toward the FDA remains cautiously optimistic: most companies report no disruption in interactions, and many continue to benefit from expedited pathways like Breakthrough Therapy and Priority Review. Indeed, the FDA leadership continues to express support for innovation, especially in rare diseases and cell and gene therapies.
From a therapeutic area perspective, rare disease, cardiovascular/metabolic, and oncology remain core focuses. We have spoken previously about our excitement around next-generation drugs to fight obesity, with consensus estimates of over $100 billion per year in peak sales. We are also increasingly excited about the opportunity within neuropsychology, particularly addressing hard-to-treat conditions with psychedelic medicine - more on that can be found in our "Strategy in Action" section later in this report.
Financial Highlights, Performance Drivers and Significant Events
Table 1. Financial Highlights in the period
| As at 30 June 2025 | Change over period | As at 31 December 2024 | As at 30 June 2024 |
Net asset value (Ordinary Shares) | $561.0 million | (6.0%) | $606.9 million | $655.4 million |
Net asset value per Ordinary Share | $1.70 | (6.0%) | $1.81 | $1.95 |
Ordinary Share price | $1.21 | (13.6%) | $1.40 | $1.55 |
Share price discount to Net Asset Value | (28.8%) | (6.0%) | (22.8%) | (20.9%) |
Number of Ordinary Shares in issue | 330.1 million | (1.7%) | 335.7 million | 335.7 million |
Russell 2000 Biotech Index | (11.4%) | |||
Nasdaq Biotech Index | (1.9%) | |||
AIC Biotechnology & Healthcare Sector | (0.2%) | |||
Figure 5. Historical performance characteristics
RTW Investments, LP, a leading global healthcare-focused investment firm with a strong track record of supporting companies developing life-changing therapies, created the Group as an investment fund focused on identifying transformative assets with high growth potential across the biopharmaceutical and medical technology sectors. Driven by deep scientific expertise and a long-term approach to building and supporting innovative businesses, we invest in companies developing transformative next-generation therapies and technologies that can significantly improve patients' lives while creating significant value for our shareholders.
RTW Bio delivered a NAV per Ordinary Share return of -6.0% during the period under review, outperforming the -11.4% from the Russell 2000 Biotech Index and underperforming the -1.9% for the Nasdaq Biotech Index. On a longer-term basis, RTW Bio continues to deliver performance ahead of the indices and peer group, with the NAV per Ordinary Share compounding at 8.8% per annum since launch.
In the first half of the year, public investments contributed -2.9%, private investments contributed -2.3%, and Royalties contributed 0.2%. Further commentary on the performance of each part of the portfolio is provided below, with specific commentary for the top contributors / detractors where the impact on NAV per Ordinary Share was >1%.
Public Investments
The Group's public investments represent the largest portion of the portfolio by design. Biotech companies typically require significant capital to fund development through key clinical milestones, and accessing the public markets is often the only viable route to secure funding at the necessary scale. While RTW is a full life cycle investor, capable of investing at any stage of a company's development, we believe that the majority of value creation in biotech occurs in the public markets. Many of our public holdings started as private positions, which we have retained in the public markets to capture as much value as possible.
Akero and UroGen were the largest contributors amongst the public positions: Akero was the strongest contributor, with its share price rising 91.8% and adding 3.8% to NAV per Ordinary Share. The company reported positive clinical data for its lead asset, efruxifermin (EFX), in MASH, a progressive liver disease with limited treatment options. Shortly after, market speculation emerged around a potential strategic acquisition, further boosting investor sentiment.
UroGen also performed well, contributing 1.5% to NAV. The company secured FDA approval for ZUSDURITM, a non-surgical treatment for a common form of bladder cancer, complementing its existing commercial product, Jelmyto®. This regulatory milestone helped drive a 28.6% increase in the share price over the period.
Rocket and Dyne were the largest detractors amongst the public positions: Rocket was the largest detractor, with its share price falling 80.5% and reducing NAV per Ordinary Share by -4.4%. The decline followed a Serious Adverse Event (SAE) and subsequent death of a patient in its pivotal Phase 2 trial for RP-A501 in Danon disease. Rocket actively engaged with the FDA and the clinical hold was subsequently released in post period end. Phase 2 trials were restarted with a recalibrated dose and a revised immunomodulatory regimen. Rocket also continues to advance a broader pipeline targeting rare genetic cardiomyopathies and haematologic disorders.
In January Dyne's shares fell after the release of updated data from its Phase 1/2 ACHIEVE trial for DYNE-101, a treatment for myotonic dystrophy type 1 (DM1). While the data were positive, investors reacted negatively to the magnitude of the improvements. Additionally in June, Dyne's revised accelerated approval strategy for DM1 drove a delay in its regulatory timeline, also contributing to negative sentiment, and reducing NAV per Ordinary Share during the period by -2.0%.
Private Investments
We primarily focus on mid-to-late-stage venture and crossover rounds, where we anticipate a company will go public within 6 to 18 months of our initial investment. As a leading crossover investor, RTW is frequently approached by high-quality private biotech companies preparing for public market entry. A smaller portion of the portfolio is allocated to early-stage ventures and RTW-founded companies, where we expect a public listing within three to five years. This balanced approach allows us to support innovation across the development lifecycle while maintaining a clear path to liquidity and value capture.
Among the Company's private investments, Corxel was the top contributor, adding 1.1% to the NAV per Ordinary Share. Corxel is an RTW company creation that is developing CX11, an oral, once-daily GLP-1 receptor agonist for obesity. In June, it enrolled the first patient in its U.S. Phase 2 trial, following positive results from a China Phase 2 trial conducted by partner Vincentage, where CX11 showed strong weight loss efficacy and a favourable safety profile. Topline data from the U.S. trial is expected in H1 2026. During the period, Corxel also received a dividend distribution linked to the sale of Aficamten to Sanofi, of which RTW Bio received its pro rata share.
Artios was the largest detractor at -3.0%. In May, Artios announced encouraging Phase 1/2a data from its lead clinical oncology candidate, ART0380. Importantly, this data hits multiple indications, meaning that development costs (and therefore capital requirements) will likely be higher than expected. We remain positive on the promising clinical trial data from ART0380, which offers the potential to transform outcomes for individuals with hard-to-treat cancers, an area of high unmet need.
Since admission, the Group has made 73 private investments. At 30 June 2025, 33 of these positions had had liquidity events (i.e., go-public or acquisition). The average holding period as private was 17 months and the average MOIC to the liquidity event was 1.7x. 23 of these positions have either concurrently or subsequently been exited in full at an average MOIC of 2.3x.
Royalty Investments
Royalty financing is a growing source of capital for life sciences companies, particularly those launching first-in-class or best-in-class therapies. It involves providing an upfront payment in exchange for a pre-determined percentage of future revenues from a specified product or asset, typically paid out as quarterly cash flows. This structure offers companies a bespoke, non-dilutive alternative to traditional debt, while aligning investor and company interests through revenue participation. RTW Bio mostly gains exposure to royalty financing through the RTW Investments-managed 4010 Royalty Fund (4010). RTW Investments' full life cycle platform and in-house transactional capabilities allow it to identify royalty opportunities early, underwrite with deep insight, and structure deals that offer robust downside protection for investors.
The Group's royalty positions, representing 2.8% of NAV, added a modest amount to the NAV per Ordinary Share. These positions comprise direct royalty investments and the Group's investment in 4010. The underlying assets have performed well in the first half of 2025. Sales of Lumryz, marketed by Avadel Pharmaceuticals, have continued to perform well and revenue guidance has been increased. Avadel also reported that the United States Court of Appeals for the Federal Circuit ruled in favor of Avadel, overturning important parts of a prior injunction, allowing Lumryz to seek approval for the treatment of indications beyond the narcolepsy model.
The Jelmyto royalty asset, marketed by UroGen Pharma, also continues to perform well. UroGen maintained its 2025 sales growth guidance for Jelmyto at 8-12% growth. The company reported long-term follow-up from the OLYMPUS trial that showed Jelmyto's median duration of response to be 48 months, adding positive long-term validation to Jelmyto's already strong clinical profile. On 12 June UroGen's UGN-102 asset received approval from the FDA and began to be marketed as Zusduri from 1 July. The RTW royalty contract with UroGen receives payments from the sales of both drugs.
Looking forward, RTW Bio is exposed to an exciting pipeline of opportunities including with Milestone Pharmaceuticals, centred on future royalty streams of its lead asset, Cardamyst, a nasal spray medication to treat a type of arrhythmia (paroxysmal supraventricular tachycardia, PSVT). Funding of the royalty is contingent on FDA approval, which is expected in the second half of the year. At the top of the sourcing funnel, we have been actively building out our pipeline by leveraging our strong network. We have several term sheets outstanding and are active in negotiations with a number of promising opportunities. We believe the current complex macro environment and elevated volatility provide an additional tailwind for the royalty value proposition as companies seek new non-dilutive capital to fund their commercial launch activities.
Figure 6. Performance breakdown in H1 2025
Portfolio Breakdown and New Investments
Our investment approach is defined as full life cycle and, therefore, involves retaining private investments beyond their IPOs; hence the portfolio consists of both privately-held and publicly-listed companies and royalty investments.
As discussed in the Chair's statement, going forward, all listed holdings will be reported under a single public category, alongside private and royalties. As of 30 June 2025, the number of holdings in each of the Group's portfolio buckets was as follows:
Figure 7. NAV capital breakdown as of 30 June 2025 compared to 30 June 2024
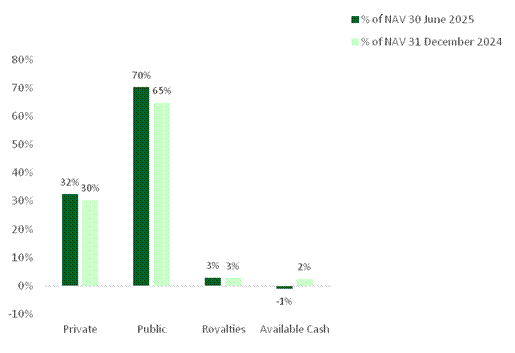
In prior periods exposures were presented as fair market value as a % of NAV and summed to 100%. In the current period they are presented as exposure as a % of NAV.
Private investments accounted for 32.4% of NAV across 36 investments. The increase in exposure from 30.3% (as at 31 December 2024) reflects five new investments over the period. These included American Laboratories (1.4% of NAV), which specialises in the production of enzymes, proteins, and flavours used across several industries, and Prolium Bioscience (1.3% of NAV), a new RTW company creation focused on autoimmune diseases.
Public investments accounted for 70.2% of NAV. The increase in exposure from 64.6% (as at 31 December 2024) reflects two privately-held positions going public. In January, Beta Bionics went public and raised gross proceeds of $204 million, trading under the ticker "BBNX". In April, Jade Biosciences completed a reverse merger with Aerovate Therapeutics and now trades on the NASDAQ under ticker "JBIO".
Royalties accounted for 2.8% of NAV across 2 investments: 4010 Royalty Fund (an RTW-managed private fund) and RTW Royalty 2 (a royalty based on the revenues of UroGen's Jelmyto and ZUSDURI).
Our "full life cycle" portfolio is diversified across clinical stages, capital position (i.e., equity and royalty), treatment modalities, and therapeutic focus giving it multiple, differentiated return levers and horizons. By constructing the portfolio in such a way, investors get exposure to the most innovative parts of a highly specialised sector with the explosive potential of companies that successfully navigate clinical, regulatory or commercial inflection points.
Looking forward, we expect the total portfolio sector allocation to remain close to 80% biopharmaceutical assets and 20% medical technology assets. In line with prospectus guidance, we anticipate two-thirds of new private investments will be made in mid- to later-stage venture companies and one-third focused on active company building around the discovery and development or licensing and distribution of promising assets. Royalty investments will be limited to approximately 15% of NAV.
Table 2. Top ten positions as of 30 June 2025
Portfolio Company | Description | Ticker | Therapeutic Area | Clinical Stage | Expected upcoming catalyst | % NAV¹ |
Corxel | RTW-incubated biotech company committed to bringing innovative therapies to underserved patients with cardiometabolic diseases. | Private | Metabolic | Phase 3 | CX11 P2 data H1 2026 | 9.4% |
Avidity | Antibody conjugated RNA medicines company. Lead program for myotonic dystrophy. | RNA | Rare Disease | Phase 3 | FSHD BLA H2 2026 | 8.8% |
Akero | Clinical-stage company developing treatments for patients with serious metabolic diseases, including non-alcoholic steatohepatitis. | AKRO | Metabolic | Phase 3 | P3 data H1 2026 | 5.2% |
Kailera | RTW co-incubated biopharma developing broad pipeline to treat obesity and related metabolic conditions. | Private | Metabolic | Phase 3 | FDA response on KAI9531 Q3 2025 | 5.0% |
PTC | Developing medicines for people living with rare neurologic and metabolic conditions disorders. | PTCT | Rare disease | Commercial | Translarna FDA decision | 5.0% |
UroGen | Biotech company developing innovative solutions to treat urothelial and specialty cancers. | URGN | Oncology | Commercial | UGN-103 P3 data in 2026 | 4.6% |
Zai Lab | Commercial stage biopharma developing medicines for oncology, immunology, neuroscience, and infectious diseases. | ZLAB | Inflammation & Immunology | Commercial | Bema P3 H2 2026 | 3.7% |
Uniqure | Advancing a pipeline of gene therapies for the treatment of Huntington's disease, refractory temporal lobe epilepsy, ALS, Fabry disease, and other severe disease. | QURE | Neurology | Registrational | 3 yr AMT-130 data in Q3 | 3.4% |
Taysha | Developing gene therapies for the treatment of monogenic diseases of the central nervous system. | TSHA | Neurology | Phase 1 | P1/2 data in Q4 | 3.1% |
Madrigal | Developed the first FDA approved treatment for metabolic dysfunction-associated steatohepatitis (MASH) | MDGL | Metabolic | Commercial | Q3 Rezdiffra earnings | 3.1% |
1 Positions are shown on a net basis. Any differences with the Schedule of Investments are due to short holdings and warrants.
Table 3. Private investments greater than 1% exposure added to the portfolio in the first half of 2025
Company name | Description | % NAV |
American Laboratories Inc | Produce and supply enzymes, protein extracts, and flavours/palatants for use across a variety of industries. | 1.4% |
Prolium Bioscience | Biotech company developing bispecific antibody programs for autoimmune diseases. | 1.3% |
Figure 8. Breakdown of portfolio positions greater than 1% exposure as a percentage of NAV, adjusted to be out of 100%, by (A) Therapeutic Focus, (B) Modality, (C) Clinical Stage and (D) Geography as of 30 June 2025. Therapeutic Focus, Modality and Geography do not include royalty vehicles.
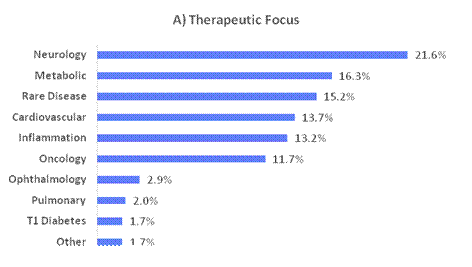
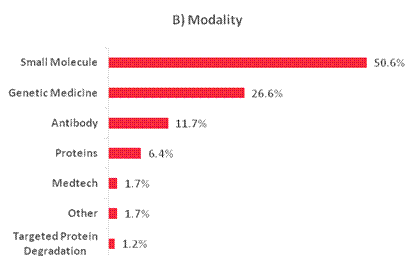
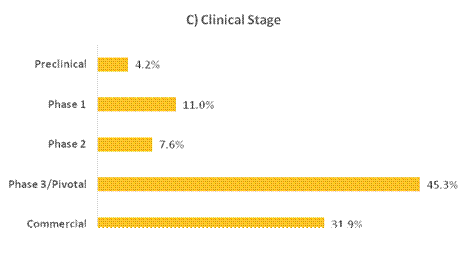
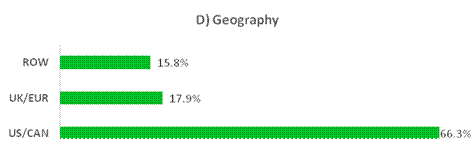
Table 4. Private positions greater than 1% exposure as of 30 June 2025 and 31 December 2024
Portfolio Company | Description | Therapeutic Area | Location | $ Position Size | % NAV 30/06/2025 | % NAV¹ 31/12/2024 |
Corxel | RTW-incubated biotech company committed to bringing innovative therapies to underserved patients with cardiometabolic diseases. | Metabolic | China | $54,732,832
| 9.4% | 8.5% |
Kailera | RTW co-incubated biopharma developing broad pipeline to treat obesity and related metabolic conditions. | Metabolic | US/CAN | $29,133,393
| 5.0% | 3.4% |
Ensoma | Genomic medicines company developing in vivo treatments that engineer any cell of the hematopoietic system for immuno-oncology and genetic diseases. | Rare Disease | US/CAN | $14,726,779
| 2.5% | 2.6% |
Artios | Developing breakthrough cancer treatments that target DNA Damage Response pathways. | Oncology | UK/EUR | $13,734,375
| 2.3% | 4.9% |
American Laboratories | Produce and supply enzymes, protein extracts, and flavours/palatants for use across a variety of industries. | Other | US/CAN | $8,250,000
| 1.4% | - |
Prolium | Biotech company developing bispecific antibody programs for autoimmune diseases. | Inflammation & Immunology | US/CAN | $7,733,529
| 1.3% | - |
Evommune | Biotech company developing therapies to treat immune-mediated chronic inflammatory diseases. | Inflammation & Immunology | US/CAN | $7,647,401
| 1.3% | 1.0% |
Lycia | Developing LYTAC degraders, a platform that can selectively reduce the levels of dysregulated proteins in blood or on cell surfaces. | Inflammation & Immunology | US/CAN | $5,856,069
| 1.0% | 1.0% |
Total > 1% | 24.2% | |||||
Total < 1% | 8.2% | |||||
Total Private | 32.4% |
1 Positions are shown on a net basis. Any differences with the Schedule of Investments are due to short holdings and warrants.
Table 5. Public positions greater than 1% exposure as of 30 June 2025 and 31 December 2024
Portfolio Company | Ticker | Description | Therapeutic Area | Location | $Position Size | % NAV 30/06/2025 | % NAV ¹ 31/12/2024 |
Avidity2 | RNA | Antibody conjugated RNA medicines company. Lead program for myotonic dystrophy. | Rare Disease | US/CAN | $51,195,466 | 8.7% | 7.3% |
Akero | AKRO | Clinical-stage company developing treatments for patients with serious metabolic diseases, including non-alcoholic steatohepatitis. | Metabolic | US/CAN | $30,167,656 | 5.2% | 5.2% |
PTC | PTCT | Developing oral small molecule drugs and gene therapy which regulate gene expression by targeting post-transcriptional control mechanisms in orphan diseases. | Neurology | US/CAN | $28,983,365 | 5.0% | 2.0% |
UroGen | URGN | Biotech company developing innovative solutions to treat urothelial and specialty cancers. | Oncology | Israel | $27,172,625 | 4.6% | 0.4% |
Zai Lab | ZLAB | Commercial stage biopharma developing medicines for oncology, immunology, neuroscience, and infectious diseases. | Inflammation & Immunology | China | $21,409,263 | 3.7% | 0.3% |
Uniqure | QURE | Gene therapy company advancing therapies for patients with severe medical needs. | Gene therapy | US/CAN | $19,878,942 | 3.4% | 0.1% |
Madrigal | MDGL | Developed the first FDA approved treatment for metabolic dysfunction-associated steatohepatitis (MASH) | Metabolic | US/CAN | $17,882,695 | 3.1% | 5.9% |
Taysha | TSHA | Focused on advancing adeno-associated virus (AAV)-based gene therapies for severe monogenic diseases of the central nervous system. | Neurology | US/CAN | $18,220,092 | 3.1% | 0.1% |
Stoke | STOK | Restoring protein expression by harnessing the body's potential with RNA medicine. | Oligo | US/CAN | $16,014,525 | 2.7% | 2.3% |
Tarsus | TARS | Biotech developing therapeutics for ophthalmic conditions. | Ophthalmology | US/CAN | $13,889,163 | 2.4% | 6.0% |
Protagonist | PTGX | Biopharma with a proprietary platform developing peptide-based new chemical entities (NCEs) that can potentially transform existing treatment paradigms in disease areas with significant unmet medical need. | Inflammation & Immunology | US/CAN | $13,872,051 | 2.4% | 1.3% |
GH Research | GHRS | Clinical-stage pharmaceutical company developing a potentially practice-changing treatment in depression. | Neurology | UK/EUR | $10,859,925 | 1.9% | 0.4% |
Milestone | MIST | Developing a chemical entity calcium channel blocker delivered as a nasal spray, for the acute at-home treatment of patients living with the heart rhythm disturbances of PSVT and AFib-RV. | Cardiovascular | US/CAN | $10,234,590 | 1.7% | 1.7% |
Verona | VRNA | Developing medicines for chronic lung conditions such as COPD, asthma, and cystic fibrosis. | Pulmonary | US/CAN | $9,358,124 | 1.6% | 0.4% |
Beta Bionics | BBNX | Develops and sells the iLet Bionic Pancreas, a fully automated insulin delivery system for people with type 1 diabetes | Type 1 Diabetes | US/CAN | $8,258,665 | 1.4% | 1.5% |
Acadia | ACAD | Developing therapies for central nervous system disorders. | Neurology | US/CAN | $8,248,864 | 1.4% | 1.1% |
Verastem | VSTM | Biotech developing drugs to treat certain cancers. It has one approved product, duvelisib, for blood cancers. | Oncology | US/CAN | $7,979,912 | 1.4% | 0.0% |
Merus | MRUS | Using its Multiclonics platform to create antibody-based therapies for various cancers. | Oncology | UK/EUR | $6,392,478 | 1.1% | 0.8% |
Rocket | RCKT | Gene therapy platform company for rare paediatric diseases. Four clinical programs for Fanconi anaemia, Danon, LAD, and PKD | Rare Disease | US/CAN | $6,272,692 | 1.1% | 5.1% |
Apogee | APGE | Developing novel antibody therapeutics for atopic dermatitis, asthma, eosinophilic esophagitis and other I&I conditions. | Inflammation & Immunology | US/CAN | $6,076,335 | 1.0% | 1.0% |
Total>1% Total Total Public | 56.9% 13.3% 70.2% |
1 Positions are shown on a net basis. Any differences with the Schedule of Investments are due to short holdings and warrants.
2 Consists primarily of common stock and pre-funded warrants.
Table 6. Royalty positions greater than 1% exposure as of 30 June 2025 and 31 December 2024
Portfolio Company | Description | Therapeutic Area | Location | $Position Size | % NAV 30/06/2025 | % NAV 31/12/2024 |
4010 Royalty | Private RTW-managed fund aiming to generate returns from rights to royalty stream distributions from biopharma and medtech life sciences companies. | Various | US/CAN | $23,892,852 | 2.0% | 2.0% |
Total > 1% | 2.0% | |||||
Total < 1% | 0.8% | |||||
Total Royalty | 2.8% |
Private Portfolio Valuations and Cash Runway Analysis
Private investments remain a core component of the Group's strategy, built on our disciplined, bottom-up approach to identifying high-quality opportunities. While we continue to maintain a selective and well-funded private portfolio, the recent dislocation in public markets has shifted the relative attractiveness of deploying new capital, as depressed public valuations create a compelling entry point for new investments. This dynamic is informing our capital allocation decisions, even as we remain committed to supporting our private companies through clinical development and strategic growth.
As of 30 June 2025, the average cash runway across our private companies was slightly over 18 months, providing sufficient time to advance clinical development plans. Around one-fifth of the portfolio had less than six months of runway; three of these are RTW Investments company creations, where the Investment Manager has the flexibility to inject capital when needed. The remainder are actively pursuing capital raising solutions.
We value our private investments at 'fair value' in accordance with US GAAP, using techniques aligned with the International Private Equity and Venture Capital ("IPEV") Guidelines. RTW Investments' Valuation Committee updates valuations on a monthly basis and in response to trigger events, aiming to ensure both timeliness and accuracy. At least twice per annum, the Valuation Committee receives the advice of an independent third-party valuation firm. Oversight is provided by the Board's Audit Committee, which reviews the Investment Manager's valuation policies and procedures semi-annually and as needed.
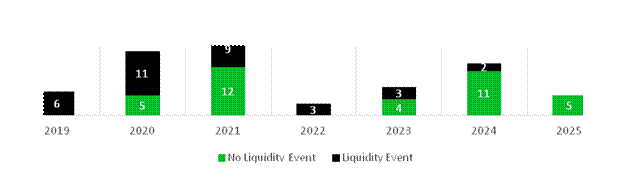
As at 30 June, RTW Bio held 38 private investments and royalty positions. During the first half of 2025, 30 private or royalty positions saw a total of 42 valuation adjustments, with an average of one adjustment per position:
· 13 positions were marked up by an average of 7.8%
· 17 positions were marked lower by an average of 21.7%
· The balance remained unchanged
The average valuation change was -6.4%. 53% of the mark-downs were primarily driven by changes to relative comparables or market-based inputs. 15% of the mark-ups were primarily driven by comparables, and 85% were primarily driven by idiosyncratic company performance, a financing round or transaction. At period end, the average time since the last third-party valuation was four weeks and an average of 14 months had elapsed since the last financing round.
Figure 9. Average Multiple of Invested Capital (MOIC) by vintage on 1) privates to liquidity event, 2) fully exited positions and 3) privates with no liquidity event
Summary of Vintages 2019-2025 | |
Number of Investments³ | 71 |
Average MOIC² | 1.6x |
Average IRR | 15.8% |
Average Hold Period to Liquidity Event¹ | 1.4 years |
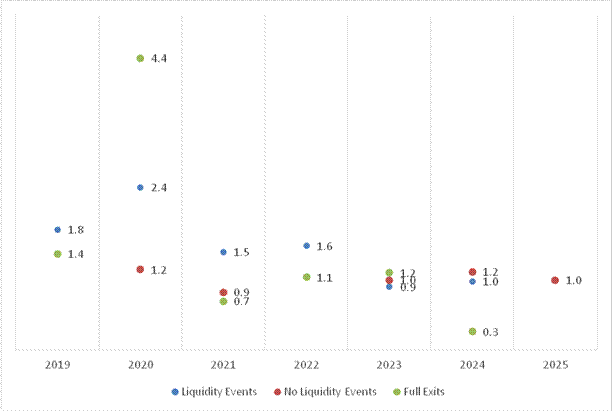
1 Liquidity event = IPO, SPAC merger, reverse merger, acquisition from private
2 Multiple of Invested Capital ("MOIC") represents the ratio of total value to the corresponding amount of total capital invested, expressed
as a multiple. Gross MOIC is utilised, which is calculated before giving effect to management fees, carried interest, taxes and other
expenses, which would reduce performance and the rate of return.
3 Certain legacy investments acquired in the 2024 Arix transaction are not included in the MOIC and IRR statistics above.
Figure 10. Private portfolio cash runway as of 30 June 2025
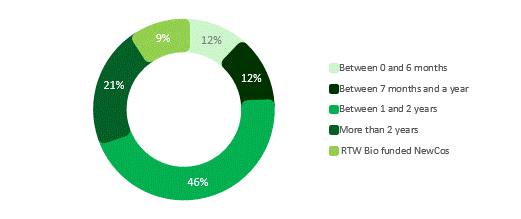
Table 7. Private Valuation Statistics for H1 2025
Statistic | H1 2025 |
Number of revaluations | 42 |
Average time since last third-party valuation | 4 weeks |
Average time since last financing round | 1.2 years |
Average valuation change | -6.4% |
Average write-up | +8.4% |
Average write-down | -21.1% |
Average MOIC to go-public event1 | 1.0x |
1 Includes one reverse merger and one IPO.
Post period-end updates and other key portfolio company events
· | Merck entered into a definitive agreement to acquire UK-based public portfolio company Verona Pharma Plc (NASDAQ: VRNA) for $107 cash per American Depositary Share, for a total transaction value of approximately $10 billion, at a 23% premium to Verona's closing price on 8 July, prior to the announcement. The transaction is subject to Verona shareholder and regulatory approvals and is expected to close in the fourth quarter of 2025. |
· | Private portfolio company Kailera Therapeutics and its partner Hengrui Pharma announced positive topline data from the China Phase 3 clinical trial of HRS9531 in individuals living with obesity or overweight. The trial met both primary endpoints, demonstrating superior weight loss and a greater percentage of participants achieving at least 5% body weight reduction compared to placebo. The safety profile was favourable and consistent with other GLP-1-based treatments, with most treatment-emergent adverse events being mild to moderate and gastrointestinal-related. |
· | Rocket Pharmaceuticals announced a strategic corporate reorganisation and pipeline prioritisation of its cardiovascular programs The company will implement a reduction in workforce of approximately 30%. Rocket anticipates that its existing cash resources will fund operations into 2027. It anticipates delays associated with the Fanconi Anemia (RP-L102) and Pyruvate Kinase Deficiency (RP-L301) programs. FDA approval of RP-L102 is no longer anticipated in 2026. |
RTW Investments, LP
10 September 2025
Strategy in Action
Impact Focus: The emergence of psychedelics as a transformative therapy for treatment-resistant depression.
Three million patients in the U.S. alone suffer from treatment-resistant depression (TRD). TRD refers to when a patient either doesn't respond to selective serotonin reuptake inhibitors (SSRIs, the primary approach to depression treatment) or experiences adverse events on them. If you fail two adequate lines of antidepressants, you are said to be treatment-resistant, and your odds of getting better go down significantly.
The interest in psychedelics as a potential treatment for TRD began as the war on drugs of the 1980s started to die down, and certain academics in the U.S., UK and elsewhere started studying magic mushrooms. They found in these early studies that the effects on depression (particularly of psilocybin) were profound. Academic research increased and the idea that previously "scheduled" drugs (controlled substances) could become approved medicines became more widespread. From this sprouted new biotech companies founded around new psychedelic compounds. With the emergence of more and more positive data from clinical trials, psychedelics continue to become destigmatised.
The current standard of care for TRD is Johnson & Johnson's drug, Spravato, which is esketamine in a nasal spray form that is delivered to patients in a clinic. Spravato's regimen is first given twice a week for four weeks and then once a week for four more weeks, with a maintenance dose of typically once a week. Spravato is trending to be a multi-billion dollar product, indicating a huge potential market. Psychedelics currently being studied look significantly more promising - they can be dosed less frequently (potentially once-a-month or even less frequently), and the response rates appear to be more durable.
The new White House administration has demonstrated its support for psychedelics. RFK Jr, FDA Commissioner Marty Makary and Trump's pick for Surgeon General, Casey Means have all made positive statements. The FDA is really leaning in, and they've even produced guidance documents for biotech companies to help them design a development program that the FDA is more likely to approve.
RTW Bio's portfolio has 2.7% exposure to psychedelics as of period end. GH Research, based in Dublin, is aiming to transform the treatment of psychiatric and neurological disorders and is pursuing therapies that have the potential to be highly effective, rapid acting, durable, well-tolerated, and conveniently administered for their target indications. Its lead product candidate is an inhaled version of mebufotenin for Treatment-Resistant Depression. Compass Pathways is a biotechnology company dedicated to accelerating patient access to evidence-based innovation in mental health. Its first major initiative is developing an investigational psilocybin treatment through a global phase 3 program for people living with treatment-resistant depression.
Impact Focus: Obesity developments in 2025 from Kailera Therapeutics and Corxel Pharmaceuticals
Kailera Therapeutics
5.0% of NAV (31 December 2024: 3.4%)
After launching in 2024, Kailera Therapeutics announced positive topline data from Hengrui Pharmaceutical's Phase 2 clinical trial of HRS9531, a GLP-1/GIP receptor dual agonist, in individuals with obesity or overweight. The clinical trial results showed that a once-weekly subcutaneous injection of the 8 mg dose of HRS9531 demonstrated a statistically significant 21.1% (p
Shortly after period-end, Kailera and Hengrui announced positive topline data from the China Phase 3 clinical trial of HRS9531 in individuals living with obesity or overweight. The trial met both primary endpoints, demonstrating superior weight loss and a greater percentage of participants achieving at least 5% body weight reduction compared to placebo. The safety profile was favourable and consistent with other GLP-1-based treatments, with most treatment-emergent adverse events being mild to moderate and gastrointestinal-related. Hengrui plans to submit a New Drug Application (NDA) for chronic weight management in China. Kailera is advancing KAI-9531 to global clinical trials.
Corxel Pharmaceuticals
9.4% of NAV (31 December 2024: 8.5%)
At the end of 2024 Corxel announced its acquisition of worldwide rights (excluding greater China) to CX11, an oral small molecule for obesity and diabetes. In June, Corxel announced the enrolment of the first patient in its U.S. Phase 2 trial of CX11, which will evaluate the efficacy and safety of CX11 in a U.S. population with a high proportion of obesity and diabetes. In a previous China Phase 2 trial conducted by its partner, Vincentage, CX11 had demonstrated competitive weight loss with favourable safety and tolerability in Chinese patients. The U.S. Phase 2 trial aims to minimise gastrointestinal side effects and will also evaluate a higher dose to achieve better weight reduction results. Topline data from this trial is expected in H1 2026.
Also in June, Corxel announced the positive China Phase 2 clinical results for CX11. In the China Phase 2 trial, weight reduction was significantly greater at all doses compared to the placebo. A weight reduction of at least 5% by week 16 occurred in 55% to 90% of the participants who received CX11, as compared to the 13% who received the placebo. Treatment with CX11 was associated with improvement in all weight-related and cardiometabolic metrics that were measured. Of reported adverse events, most were gastrointestinal and mild to moderate in severity. No liver toxicity safety signal was identified, nor were any drug-related serious adverse events reported.
Responsible Investment
The Group aims to achieve superior long-term capital appreciation, focusing on forming, building, and supporting world-class life sciences, biopharmaceutical, and medical technology companies. The Group's primary consideration is to support companies that promote health and well-being by bringing drugs and devices to market that are expected to save or extend life, improve quality of life, or revolutionise the course of treatment for diseases and conditions that afflict people.
The Group (via the Investment Manager) seeks to meet regularly with the management teams of portfolio companies to foster long-term relationships. This ongoing dialogue enables open discussions on issues that could affect long-term returns. The decision to engage with a portfolio company depends on both the materiality of the issue and the size of the holding.
The Group is committed to investing in and building companies that adopt and integrate sound practices that are proportionate to the scale of the business, including established industry best practices related to clinical trials and animal testing, contract research organization (CRO) oversight, anti-bribery and corruption and the development of a governance framework. On an ongoing basis, the Investment Manager's IR team engages with portfolio companies to collect information on sustainability data and policies where they exist.
The Group's Board of Directors has established a Sustainability Committee which meets with the Investment Manager bi-annually where they are briefed on updates to process, policy, and portfolio engagement. The Sustainability Committee provides oversight on behalf of and advice to the Board in relation to the Company's Responsible Investment strategy and ensures that all stakeholders receive appropriate related information. The Committee assists the Board in overseeing the development of the Responsible Investment Policy and reviews relevant matters to be presented in this half-yearly financial report, maintaining oversight of and making recommendations.
Statement of Principal Risks and Uncertainties for the Remaining Six Months of the year to 31 December 2025
As described in the Group's annual consolidated financial statements for the year ended 31 December 2024, the Group's principal and emerging risks and uncertainties include the following:
- Failure to achieve investment objective;
- Unfavourable tax exposure;
- Counterparty risk;
- The Investment Manager relies on key personnel;
- Portfolio companies may be subject to litigation;
- Exposure to global political and economic risks;
- Clinical development and regulatory risks;
- Imposition of pricing controls for clinical products and services;
- Inflation;
- Availability of capital; and
- Sustainability reporting.
The Board believes that these risks are unchanged in respect of the remaining six months of the year to 31 December 2025.
Further information in relation to these principal risks and uncertainties may be found on pages 38 to 40 of the Group's annual report and audited consolidated financial statements for the year ended 31 December 2024.
These inherent risks associated with investments in the biotech and pharmaceutical sector could result in a material adverse effect on the Group's performance and value of the Ordinary Shares.
Risks are mitigated and managed by the Board through continual review, policy setting and regular reviews of the Group's risk matrix by the Audit Committee to ensure that procedures are in place with the intention of minimising the impact of the above-mentioned risks. The Board carried out a formal review of the risk matrix at the Audit Committee meeting held on 31 July 2025. The Board relies on periodic reports provided by the Investment Manager and Administrator regarding risks that the Group faces. When required, experts will be employed to gather information and/or provide expert advice, including tax and legal advisers.
Statement of Directors' Responsibilities
The Directors confirm to the best of their knowledge that:
- the unaudited interim consolidated financial statements have been prepared in conformity with US generally accepted accounting principles and give a true and fair view of the assets, liabilities, financial position and profit or loss of the Group; and
- the interim management report (which includes the Chair's Statement, Report of the Investment Manager and Statement of Principal Risks and Uncertainties) together with the unaudited interim consolidated financial statements include a fair review of the information required by:
a. DTR 4.2.7R of the Disclosure Guidance and Transparency Rules, being an indication of important events that have occurred during the first six months of the financial year and their impact on the unaudited interim consolidated financial statements; and a description of the principal risks and uncertainties for the remaining six months of the financial year; and
b. DTR 4.2.8R of the Disclosure Guidance and Transparency Rules, being related party transactions that have taken place during the first six months of the financial year and that have materially affected the financial position or performance of the Group during that period; and any changes in the related party transactions described in the last annual report that could do so.
The Directors are responsible for the maintenance and integrity of the corporate and financial information included on the Group's website (https://www.rtwbio.com). Legislation in Guernsey governing the preparation and dissemination of financial statements may differ from legislation in other jurisdictions.
By order of the Board
William Simpson Chair 10 September 2025 | Paul Le Page Director 10 September 2025 |
Independent Review Report to RTW Biotech Opportunities Ltd
Conclusion
We have been engaged by RTW Biotech Opportunities Ltd (the "Company") to review the consolidated financial statements in the half-yearly financial report for the six months ended 30 June 2025 of the Company and its subsidiaries (together, the "Group"), which comprises the unaudited interim consolidated statement of assets and liabilities including the unaudited interim consolidated condensed schedule of investments, the unaudited interim consolidated statements of operations, changes in net assets and cash flows and the related explanatory notes.
Based on our review, nothing has come to our attention that causes us to believe that the consolidated financial statements in the half-yearly financial report for the period ended 30 June 2025 do not give a true and fair view of the financial position of the Group as at 30 June 2025 and of its financial performance and its cash flows for the six month period then ended, in accordance with U.S. generally accepted accounting principles and the Disclosure Guidance and Transparency Rules ("the DTR") of the UK's Financial Conduct Authority ("the UK FCA").
Scope of review
We conducted our review in accordance with International Standard on Review Engagements (UK) 2410 Review of Interim Financial Information Performed by the Independent Auditor of the Entity ("ISRE (UK) 2410") issued by the Financial Reporting Council for use in the UK. A review of interim financial information consists of making enquiries, primarily of persons responsible for financial and accounting matters, and applying analytical and other review procedures. We read the other information contained in the half-yearly financial report and consider whether it contains any apparent misstatements or material inconsistencies with the information in the consolidated financial statements.
A review is substantially less in scope than an audit conducted in accordance with International Standards on Auditing (UK) and consequently does not enable us to obtain assurance that we would become aware of all significant matters that might be identified in an audit. Accordingly, we do not express an audit opinion.
Conclusions relating to going concern
Based on our review procedures, which are less extensive than those performed in an audit as described in the Scope of review section of this report, nothing has come to our attention to suggest that the directors have inappropriately adopted the going concern basis of accounting or that the directors have identified material uncertainties relating to going concern that are not appropriately disclosed.
This conclusion is based on the review procedures performed in accordance with ISRE (UK) 2410. However future events or conditions may cause the Group and the Company to cease to continue as a going concern, and the above conclusions are not a guarantee that the Group and the Company will continue in operation.
Directors' responsibilities
The half-yearly financial report is the responsibility of, and has been approved by, the directors. The directors are responsible for preparing the interim financial report in accordance with the DTR of the UK FCA.
The consolidated financial statements included in this interim report have been prepared in accordance with U.S. generally accepted accounting principles.
In preparing the half-yearly financial report, the directors are responsible for assessing the Group and the Company's ability to continue as a going concern, disclosing, as applicable, matters related to going concern and using the going concern basis of accounting unless liquidation is imminent.
Our responsibility
Our responsibility is to express to the Company a conclusion on the consolidated financial statements in the half-yearly financial report based on our review. Our conclusion, including our conclusions relating to going concern, are based on procedures that are less extensive than audit procedures, as described in the scope of review paragraph of this report.
The purpose of our review work and to whom we owe our responsibilities
This report is made solely to the Company in accordance with the terms of our engagement letter to assist the Company in meeting the requirements of the DTR of the UK FCA. Our review has been undertaken so that we might state to the Company those matters we are required to state to it in this report and for no other purpose. To the fullest extent permitted by law, we do not accept or assume responsibility to anyone other than the Company for our review work, for this report, or for the conclusions we have reached.
Andrew J. Salisbury
For and on behalf of KPMG Channel Islands Limited
Chartered Accountants
Guernsey
10 September 2025
Unaudited Interim Consolidated Statement of Assets and Liabilities
as at 30 June 2025 and 31 December 2024
(Expressed in United States Dollars)
30 June 2025 (unaudited) |
| 31 December 2024 (audited) | ||
|
|
|
| |
ASSETS: |
|
|
| |
Investments in securities, at fair value (cost at 30 June 2025: $576,162,841; 31 December 2024: $529,516,651) |
603,470,074 |
611,011,096 | ||
Derivative contracts, at fair value (cost at 30 June 2025: $81,373,854; 31 December 2024: $60,427,785) |
118,770,502 |
110,177,172 | ||
Cash and cash equivalents | 2,661,257 | 5,360,022 | ||
Due from brokers | 39,645,459 | 27,990,478 | ||
Receivable from unsettled trades | 367,454 | 4,237,674 | ||
Other assets | 1,206,126 | 1,239,967 | ||
TOTAL ASSETS |
| 766,120,872 |
| 760,016,409 |
LIABILITIES: | ||||
Securities sold short, at fair value (proceeds at 30 June 2025: $135,080,555; 31 December 2024: $102,512,585) |
126,303,780 |
95,151,493 | ||
Derivative contracts, at fair value (proceeds at 30 June 2025: $nil; 31 December 2024: $nil) |
4,875,333 |
7,799,422 | ||
Due to brokers | 48,557,180 | 23,570,906 | ||
Payable for unsettled trades | 210,465 | - | ||
Accrued expenses | 975,177 | 850,903 | ||
TOTAL LIABILITIES | 180,921,935 | 127,372,724 | ||
| ||||
TOTAL NET ASSETS | 585,198,937 |
| 632,643,685 | |
|
|
|
| |
NET ASSETS attributable to Ordinary Shares (shares at 30 June 2025: 330,063,649; 31 December 2024: 335,713,649) | 561,014,995 |
| 606,921,161 | |
NET ASSETS attributable to Non-Controlling Interest |
| 24,183,942 |
| 25,722,524 |
|
|
|
|
|
NAV per Ordinary Share | 1.6997 | 1.8079 |
The unaudited interim consolidated financial statements of the Group were approved and authorised for issue by the Board of Directors on 10 September 2025 and signed on its behalf by:
William Simpson Paul Le Page
Chair Director
See accompanying notes to the unaudited interim consolidated financial statements.
Unaudited Interim Consolidated Condensed Schedule of Investments
as at 30 June 2025
(Expressed in United States Dollars)
Descriptions | Number of Shares |
| Cost |
| Fair Value |
| Percentage of Net Assets |
| ||
Investments in securities, at fair value | ||||||||||
Common stocks | ||||||||||
United States | ||||||||||
Healthcare | ||||||||||
Madrigal Pharmaceuticals, Inc. | 218,276 | 50,398,109 | 66,059,049 | 11.29 | ||||||
Akero Therapeutics, Inc. | 565,588 | 17,819,262 | 30,179,776 | 5.16 | ||||||
Avidity Biosciences, Inc. | 369,865 | 6,102,773 | 10,504,166 | 1.79 | ||||||
Others* | 182,793,480 | 178,653,037 | 30.53 | |||||||
Total United States | 257,113,624 |
| 285,396,028 |
| 48.77 | |||||
|
|
|
|
|
|
|
|
| ||
Netherlands |
|
|
|
|
|
|
|
| ||
Healthcare* |
|
| 27,499,147 |
| 30,017,895 |
| 5.13 |
| ||
|
|
|
|
|
|
|
|
| ||
Ireland |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 11,870,496 |
| 11,666,875 |
| 1.99 |
| ||
|
|
|
|
|
|
|
|
| ||
Switzerland |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 1,019,250 |
| 1,072,344 |
| 0.18 |
| ||
|
|
|
|
|
|
| ||||
British Virgin Islands |
|
|
|
|
|
| ||||
Healthcare |
|
| 479,464 |
| 616,903 |
| 0.11 |
| ||
|
|
|
|
|
| |||||
China |
|
|
|
|
|
| ||||
Healthcare |
|
|
|
|
|
| ||||
Corxel Pharmaceuticals Ltd. | 541,205 |
| 216,482 |
| 372,115 |
| 0.06 |
| ||
Others* |
|
| 31,835 |
| 38,401 |
| 0.01 |
| ||
Total China |
|
| 248,317 |
| 410,516 |
| 0.07 |
| ||
|
|
|
|
|
|
|
|
| ||
Canada |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 2,752,161 |
| 379,625 |
| 0.06 |
| ||
|
|
|
|
|
|
|
|
| ||
Cayman Islands |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 175,045 | 213,613 | 0.04 | |||||
|
|
|
|
|
|
|
|
| ||
Singapore |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 191,496 |
| 155,592 |
| 0.03 |
| ||
|
|
|
|
|
|
|
|
| ||
France |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 3,930,888 |
| 107,084 |
| 0.02 |
| ||
|
|
|
|
|
|
|
|
| ||
Japan |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 64,326 |
| 57,105 |
| 0.01 |
| ||
|
|
|
|
| ||||||
United Kingdom |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 4,887 |
| 0 |
| 0.00 |
| ||
|
|
|
|
| ||||||
Total common stocks |
|
| 305,349,101 |
| 330,093,580 |
| 56.41 |
| ||
|
|
|
|
|
|
|
|
| ||
* No individual investment security or contract constitutes greater than 5 per cent. of net assets. | ||||||||||
See accompanying notes to the unaudited interim consolidated financial statements.
Descriptions | Number of Shares |
| Cost |
| Fair Value |
| Percentage of Net Assets |
| ||
Investments in securities, at fair value (continued) | ||||||||||
Convertible preferred stocks | ||||||||||
United States |
|
|
|
|
|
|
|
| ||
Healthcare* |
|
| 88,597,376 | 94,043,697 | 16.07 |
| ||||
|
|
|
|
|
|
|
|
| ||
China |
|
|
|
|
|
|
|
| ||
Healthcare |
|
|
|
|
|
|
|
| ||
Corxel Pharmaceuticals Ltd. | 14,177,776 |
| 25,664,114 | 14,997,530 | 2.56 |
| ||||
Others* |
|
| 4,110,583 | 3,661,332 | 0.62 |
| ||||
Total China |
|
| 29,774,697 |
| 18,658,862 |
| 3.18 |
| ||
|
|
|
|
|
|
|
|
| ||
United Kingdom |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 16,347,749 |
| 16,946,928 |
| 2.90 |
| ||
|
|
|
|
| ||||||
Switzerland |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 567,048 |
| 1,416,497 |
| 0.24 |
| ||
|
|
|
|
| ||||||
Netherlands |
|
|
|
|
| |||||
Healthcare |
|
| 1,166,079 |
| 1,326,043 |
| 0.23 |
| ||
|
|
|
|
| ||||||
Belgium |
|
|
|
|
| |||||
Healthcare |
|
| 0 |
| 0 |
| 0.00 |
| ||
|
|
|
|
|
|
|
|
| ||
Total convertible preferred stocks |
|
| 136,452,949 |
| 132,392,027 |
| 22.62 |
| ||
|
|
|
|
|
|
|
|
| ||
American depository receipts |
|
|
|
|
|
|
|
| ||
United Kingdom |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 23,785,683 |
| 28,729,660 |
| 4.91 |
| ||
|
|
|
| |||||||
China |
|
|
|
| ||||||
Healthcare |
| 18,162,417 |
| 21,409,263 |
| 3.66 |
| |||
|
|
|
| |||||||
Netherlands |
|
|
|
|
| |||||
Healthcare |
|
| 12,797,852 |
| 13,435,988 |
| 2.29 |
| ||
|
|
|
| |||||||
Belgium |
|
|
|
|
| |||||
Healthcare |
|
| 3,070,856 |
| 3,368,317 |
| 0.57 |
| ||
|
|
|
| |||||||
Total American depository receipts |
|
| 57,816,808 |
| 66,943,228 |
| 11.43 |
| ||
|
|
|
| |||||||
|
|
|
|
|
|
|
|
| ||
* No individual investment security or contract constitutes greater than 5 per cent. of net assets. | ||||||||||
|
|
|
|
|
|
|
|
| ||
See accompanying notes to the unaudited interim consolidated financial statements.
|
|
|
|
|
|
|
|
| |
Descriptions | Number of Shares |
| Cost |
| Fair Value |
| Percentage of Net Assets |
| |
|
|
|
|
|
| ||||
Investments in securities, at fair value (continued) |
|
|
|
|
|
| |||
|
|
|
|
|
|
|
|
| |
Convertible Notes |
|
|
|
|
|
|
|
| |
China |
|
|
|
|
|
|
|
| |
Healthcare |
|
|
|
|
|
|
|
| |
Corxel Pharmaceuticals Ltd. | 3,766,269 |
| 37,662,686 |
| 39,363,186 |
| 6.73 |
| |
|
|
|
|
|
|
|
|
| |
United States |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 13,325,758 |
| 8,726,138 |
| 1.50 |
| |
|
|
|
|
|
|
|
|
| |
Canada |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 7,512,664 |
| 7,978,607 |
| 1.36 |
| |
|
|
|
|
|
|
|
|
| |
British Virgin Islands |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 161,610 |
| 162,149 |
| 0.03 |
| |
|
|
|
|
|
|
|
|
| |
Total convertible notes |
|
| 58,662,718 |
| 56,230,080 |
| 9.62 |
| |
|
|
|
|
|
| ||||
Investment in private investment companies |
|
|
|
| |||||
Cayman Islands |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 10,427,832 |
| 11,556,676 |
| 1.97 |
| |
|
|
|
|
|
|
|
|
| |
Ireland |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 3,221,986 |
| 4,704,360 |
| 0.80 |
| |
|
|
|
|
| |||||
United Kingdom |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 4,064,686 |
| 1,363,213 |
| 0.24 |
| |
|
|
|
|
|
|
|
|
| |
Total investment in private investment companies |
|
| 17,714,504 |
| 17,624,249 |
|
3.01 |
| |
|
|
|
|
|
| ||||
Revenue based financing agreement |
|
|
|
|
|
|
|
| |
United States |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 160,731 |
| 186,910 |
| 0.03 |
| |
|
|
|
|
|
| ||||
Corporate bonds |
|
|
|
|
|
|
|
| |
Bermuda |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 6,030 |
| 0 |
| 0.00 |
| |
|
|
|
|
|
|
|
|
| |
Total investments in securities, at fair value |
| 576,162,841 |
| 603,470,074 |
| 103.12 |
| ||
|
|
|
|
|
|
|
|
| |
See accompanying notes to the unaudited interim consolidated financial statements. | |||||||||
|
|
|
|
|
|
|
|
| ||
Descriptions | Number of contracts |
| Cost |
| Fair Value |
| Percentage of Net Assets |
| ||
Derivative contracts - assets, at fair value | ||||||||||
Warrants |
|
|
|
|
|
|
|
| ||
United States | ||||||||||
Healthcare | ||||||||||
Avidity Biosciences, Inc. | 2,208,114 | 36,431,673 | 62,708,229 | 10.72 | ||||||
Akero Therapeutics, Inc. | 68,710 | 3,298,073 | 3,666,359 | 0.63 | ||||||
Others* | 36,439,696 | 25,363,749 | 4.33 | |||||||
Total United States | 76,169,442 |
| 91,738,337 |
| 15.68 | |||||
Canada | ||||||||||
Healthcare | 3,121,272 | 1,876,358 | 0.32 | |||||||
British Virgin Islands | ||||||||||
Healthcare | 1,349,970 | 1,261,368 | 0.22 | |||||||
United Kingdom | ||||||||||
Healthcare | 101,902 | 83,181 | 0.01 | |||||||
Total warrants | 80,742,586 |
| 94,959,244 |
| 16.23 | |||||
| ||||||||||
Equity swaps | ||||||||||
United States | ||||||||||
Healthcare | ||||||||||
Avidity Biosciences, Inc. | 15,207 | 3,036 | 0.00 | |||||||
Others* | 21,968,139 | 3.75 | ||||||||
Total United States |
|
| 21,971,175 |
| 3.75 | |||||
Netherlands | ||||||||||
Healthcare | 58,926 | 0.01 | ||||||||
British Virgin Islands | ||||||||||
Healthcare | 51,131 | 0.01 | ||||||||
Japan | ||||||||||
Healthcare | 32,301 | 0.01 | ||||||||
Total equity swaps |
|
| 22,113,533 |
| 3.78 |
| ||||
* No individual investment security or contract constitutes greater than 5 per cent. of net assets. | ||||||||||
See accompanying notes to the unaudited interim consolidated financial statements.
|
|
|
|
|
|
|
|
| ||
Descriptions | Number of contracts |
| Cost |
| Fair Value |
| Percentage of Net Assets |
| ||
Derivative contracts - assets, at fair value (continued) | ||||||||||
Contingent value rights |
|
|
|
|
|
|
|
| ||
United States | ||||||||||
Healthcare | 466,420 | 973,334 | 0.17 | |||||||
Switzerland | ||||||||||
Healthcare | 164,848 | 724,391 | 0.12 | |||||||
Denmark | ||||||||||
Healthcare | 0 | 0 | 0.00 | |||||||
Total contingent value rights | 631,268 |
| 1,697,725 |
| 0.29 | |||||
Total derivative contracts - assets, at fair value |
| 81,373,854 |
| 118,770,502 |
| 20.30 |
| |||
| ||||||||||
See accompanying notes to the unaudited interim consolidated financial statements.
Descriptions |
|
|
| Proceeds |
| Fair Value |
| Percentage of Net Assets |
| ||
Securities sold short, at fair value | |||||||||||
Common stocks | |||||||||||
| United States | ||||||||||
Healthcare | |||||||||||
Madrigal Pharmaceuticals, Inc. | 159,187 | 43,184,890 | 48,176,354 | 8.23 | |||||||
Others* | 80,976,946 | 67,364,850 | 11.51 | ||||||||
Total United States | 124,161,836 |
| 115,541,204 |
| 19.74 | ||||||
Singapore | |||||||||||
Healthcare | 200,738 | 155,590 | 0.03 | ||||||||
| |||||||||||
Total common stocks | 124,362,574 |
| 115,696,794 |
| 19.77 |
| |||||
|
|
|
|
|
|
| |||||
American depository receipts | |||||||||||
| Netherlands | ||||||||||
Healthcare | 7,401,056 | 6,993,328 | 1.20 | ||||||||
United Kingdom | |||||||||||
Healthcare | 3,316,925 | 3,613,658 | 0.61 | ||||||||
| |||||||||||
Total American depository receipts | 10,717,981 |
| 10,606,986 |
| 1.81 |
| |||||
|
|
|
|
| |||||||
Total securities sold short, at fair value |
|
| 135,080,555 |
| 126,303,780 |
| 21.58 |
| |||
Descriptions |
|
|
| Fair Value |
| Percentage of Net Assets |
| ||
Derivative contracts - liabilities, at fair value | |||||||||
Equity swaps | |||||||||
| United States | ||||||||
Healthcare | 2,711,672 | 0.46 | |||||||
Ireland | |||||||||
Healthcare | 1,686,193 | 0.29 | |||||||
United Kingdom | |||||||||
Healthcare | 396,479 | 0.07 | |||||||
British Virgin Islands | |||||||||
Healthcare | 80,989 | 0.01 | |||||||
Total derivative contracts - liabilities, at fair value |
| 4,875,333 |
| 0.83 |
| ||||
* No individual investment security or contract constitutes greater than 5 per cent. of net assets. | |||||||||
See accompanying notes to the unaudited interim consolidated financial statements.
Audited Consolidated Condensed Schedule of Investments
as at 31 December 2024
(Expressed in United States Dollars)
Descriptions | Number of Shares |
| Cost |
| Fair Value |
| Percentage of Net Assets |
| ||
Investments in securities, at fair value | ||||||||||
Common stocks | ||||||||||
United States | ||||||||||
Healthcare | ||||||||||
Madrigal Pharmaceuticals, Inc. | 214,826 | 49,317,124 | 66,288,859 | 10.48 | ||||||
Akero Pharmaceuticals, Inc. | 1,191,010 | 26,909,569 | 33,133,898 | 5.24 | ||||||
Rocket Pharmaceuticals, Inc. | 2,400,755 | 8,188,796 | 30,177,490 | 4.77 | ||||||
Tarsus Pharmaceuticals, Inc. | 401,308 | 8,874,464 | 22,220,424 | 3.51 | ||||||
Avidity Biosciences, Inc. | 369,865 | 6,102,773 | 10,755,674 | 1.70 | ||||||
Others* | 190,069,145 | 174,522,722 | 27.58 | |||||||
Total United States | 289,461,871 |
| 337,099,067 |
| 53.28 | |||||
|
|
|
|
|
|
|
|
| ||
Netherlands |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 12,693,165 |
| 16,077,163 |
| 2.55 |
| ||
|
|
|
|
|
|
|
|
| ||
Ireland |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 10,013,472 |
| 8,557,542 |
| 1.36 |
| ||
|
|
|
|
|
|
|
|
| ||
China |
|
|
|
|
|
|
|
| ||
Healthcare |
|
|
|
|
|
|
|
| ||
Corxel Pharmaceuticals Ltd. (formerly Ji Xing Pharmaceuticals Ltd.) | 541,205 |
| 216,482 |
| 835,037 |
| 0.13 |
| ||
|
|
|
|
|
|
|
|
| ||
Canada |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 2,879,914 |
| 518,365 |
| 0.08 |
| ||
|
|
|
|
|
|
|
|
| ||
Denmark |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 301,757 | 305,536 | 0.05 | |||||
|
|
|
|
|
|
|
|
| ||
Singapore |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 191,496 |
| 296,101 |
| 0.05 |
| ||
|
|
|
|
|
|
|
|
| ||
France |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 3,930,888 |
| 79,772 |
| 0.01 |
| ||
|
|
|
|
|
|
|
|
| ||
Cayman Islands |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 77,953 |
| 73,384 |
| 0.01 |
| ||
|
|
|
|
| ||||||
Japan |
|
|
|
|
| |||||
Healthcare |
|
| 64,326 |
| 70,334 |
| 0.01 |
| ||
|
|
|
|
| ||||||
Switzerland |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 2,496 |
| 17,811 |
| 0.00 |
| ||
|
|
|
|
| ||||||
United Kingdom |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 4,992 |
| 17,413 |
| 0.00 |
| ||
|
|
|
|
| ||||||
Total common stocks |
|
| 319,838,812 |
| 363,947,525 |
| 57.53 |
| ||
|
|
|
|
|
|
|
|
| ||
* No individual investment security or contract constitutes greater than 5 per cent. of net assets. | ||||||||||
See accompanying notes to the unaudited interim consolidated financial statements.
Descriptions | Number of Shares |
| Cost |
| Fair Value |
| Percentage of Net Assets |
| ||
Investments in securities, at fair value (continued) | ||||||||||
Convertible preferred stocks | ||||||||||
United States |
|
|
|
|
|
|
|
| ||
Healthcare* |
|
| 81,802,284 | 89,628,561 | 14.17 |
| ||||
|
|
|
|
|
|
|
|
| ||
China |
|
|
|
|
|
|
|
| ||
Healthcare |
|
|
|
|
|
|
|
| ||
Corxel Pharmaceuticals Ltd. (formerly Ji Xing Pharmaceuticals Ltd.) | 14,177,776 |
| 25,664,114 |
| 34,445,874 |
| 5.44 |
| ||
Others* |
|
| 4,110,584 |
| 3,952,898 |
| 0.63 |
| ||
Total China |
|
| 29,774,698 |
| 38,398,772 |
| 6.07 |
| ||
|
|
|
|
|
|
|
|
| ||
United Kingdom |
|
|
|
|
|
|
|
| ||
Healthcare* |
|
| 16,347,749 |
| 34,368,669 |
| 5.44 |
| ||
|
|
|
|
| ||||||
Netherlands |
|
|
|
|
|
|
|
| ||
Healthcare |
|
| 1,166,079 |
| 1,165,404 |
| 0.18 |
| ||
|
|
|
|
| ||||||
Switzerland |
|
|
|
|
| |||||
Healthcare |
|
| 90,748 |
| 763,629 |
| 0.12 |
| ||
|
|
|
|
| ||||||
Belgium |
|
|
|
|
| |||||
Healthcare |
|
| 0 |
| 0 |
| 0.00 |
| ||
|
|
|
|
|
|
|
|
| ||
Total convertible preferred stocks |
|
| 129,181,558 |
| 164,325,035 |
| 25.98 |
| ||
|
|
|
|
|
|
|
|
| ||
Convertible Notes |
|
|
|
|
|
|
|
| ||
China |
|
|
|
|
|
|
|
| ||
Healthcare |
|
|
|
|
|
|
|
| ||
Corxel Pharmaceuticals Ltd. (formerly Ji Xing Pharmaceuticals Ltd.) | 1,803,339 |
| 18,033,384 |
| 18,381,736 |
| 2.91 |
| ||
|
|
|
| |||||||
Canada |
|
|
|
|
| |||||
Healthcare |
|
| 7,512,664 |
| 8,050,255 |
| 1.27 |
| ||
|
|
|
| |||||||
United States |
|
|
|
|
| |||||
Healthcare |
|
| 8,679,051 |
| 6,312,757 |
| 1.00 |
| ||
|
|
|
| |||||||
Total convertible notes |
|
| 34,225,099 |
| 32,744,748 |
| 5.18 |
| ||
|
|
|
| |||||||
|
|
|
|
|
|
|
|
| ||
* No individual investment security or contract constitutes greater than 5 per cent. of net assets. | ||||||||||
|
|
|
|
|
|
|
|
| ||
See accompanying notes to the unaudited interim consolidated financial statements.
|
|
|
|
|
|
|
|
| |
Descriptions | Number of Shares |
| Cost |
| Fair Value |
| Percentage of Net Assets |
| |
|
|
|
|
|
| ||||
Investments in securities, at fair value (continued) |
|
|
|
|
|
| |||
|
|
|
|
|
|
|
|
| |
American depository receipts |
|
|
|
|
|
|
|
| |
United Kingdom |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 16,687,163 |
| 17,163,590 |
| 2.72 |
| |
|
|
|
|
|
|
|
|
| |
Netherlands |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 9,685,018 |
| 11,905,170 |
| 1.88 |
| |
|
|
|
|
|
|
|
|
| |
China |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 1,616,703 |
| 1,602,514 |
| 0.25 |
| |
|
|
|
|
|
|
|
|
| |
Cayman Islands |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 102,795 |
| 53,101 |
| 0.01 |
| |
|
|
|
|
|
|
|
|
| |
Total American depository receipts |
|
| 28,091,679 |
| 30,724,375 |
| 4.86 |
| |
|
|
|
|
|
| ||||
Investment in private investment companies |
|
|
|
| |||||
Cayman Islands |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 10,348,706 |
| 12,571,857 |
| 1.99 |
| |
|
|
|
|
|
|
|
|
| |
Ireland |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 3,221,986 |
| 4,602,256 |
| 0.73 |
| |
|
|
|
|
| |||||
United Kingdom |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 4,444,220 |
| 1,920,687 |
| 0.30 |
| |
|
|
|
|
|
|
|
|
| |
Total investment in private investment companies |
|
| 18,014,912 |
| 19,094,800 |
|
3.02 |
| |
|
|
|
|
|
| ||||
Revenue based financing agreement |
|
|
|
|
|
|
|
| |
United States |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 160,732 |
| 174,613 |
| 0.01 |
| |
|
|
|
|
|
| ||||
Corporate bonds |
|
|
|
|
|
|
|
| |
Bermuda |
|
|
|
|
|
|
|
| |
Healthcare |
|
| 3,859 |
| 0.00 |
| 0.00 |
| |
|
|
|
|
|
|
|
|
| |
Total investments in securities, at fair value |
| 529,516,651 |
| 611,011,096 |
| 96.58 |
| ||
|
|
|
|
|
|
|
|
| |
See accompanying notes to the unaudited interim consolidated financial statements. | |||||||||
|
|
|
|
|
|
|
|
| ||
Descriptions | Number of contracts |
| Cost |
| Fair Value |
| Percentage of Net Assets |
| ||
Derivative contracts - assets, at fair value | ||||||||||
Warrants |
|
|
|
|
|
|
|
| ||
United States | ||||||||||
Healthcare | ||||||||||
Avidity Biosciences, Inc. | 2,208,114 | 36,431,673 | 64,209,747 | 10.15 | ||||||
Tarsus Pharmaceuticals, Inc. | 150,000 | 4,799,985 | 8,305,485 | 1.31 | ||||||
Rocket Pharmaceuticals, Inc. | 170,764 | 2,565,561 | 2,010,658 | 0.32 | ||||||
Others* | 11,528,056 | 9,877,117 | 1.56 | |||||||
Total United States | 55,325,275 |
| 84,403,007 |
| 13.34 | |||||
Canada | ||||||||||
Healthcare | 3,121,272 | 2,283,707 | 0.36 | |||||||
British Virgin Islands | ||||||||||
Healthcare | 1,349,970 | 1,360,602 | 0.22 | |||||||
Total warrants | 59,796,517 |
| 88,047,316 |
| 13.92 | |||||
| ||||||||||
Equity swaps | ||||||||||
United States | ||||||||||
Healthcare | ||||||||||
Tarsus Pharmaceuticals, Inc. | 215,335 | 7,603,492 | 1.20 | |||||||
Others* | 12,594,491 | 1.99 | ||||||||
Total United States |
|
| 20,197,983 |
| 3.19 | |||||
British Virgin Islands | ||||||||||
Healthcare | 328,499 | 0.05 | ||||||||
Total equity swaps |
|
| 20,526,482 |
| 3.24 |
| ||||
Contingent value rights | ||||||||||
| United States | |||||||||
| Healthcare | 466,420 | 1,023,626 | 0.17 | ||||||
| ||||||||||
| Switzerland | |||||||||
| Healthcare | 164,848 | 579,748 | 0.09 | ||||||
| ||||||||||
Total contingent value rights | 631,268 |
| 1,603,374 |
| 0.26 | |||||
Total derivative contracts - assets, at fair value |
| 60,427,785 |
| 110,177,172 |
| 17.42 |
| |||
| ||||||||||
* No individual investment security or contract constitutes greater than 5 per cent. of net assets. | ||||||||||
See accompanying notes to the unaudited interim consolidated financial statements.
Descriptions |
|
|
| Proceeds |
| Fair Value |
| Percentage of Net Assets |
| ||
Securities sold short, at fair value | |||||||||||
Common stocks | |||||||||||
| United States | ||||||||||
Healthcare* | 100,739,418 | 93,400,032 | 14.76 | ||||||||
British Virgin Islands | |||||||||||
Healthcare | 1,164,515 | 1,141,154 | 0.18 | ||||||||
Singapore | |||||||||||
Healthcare | 200,738 | 296,101 | 0.05 | ||||||||
| |||||||||||
Total common stocks | 102,104,671 |
| 94,837,287 |
| 14.99 |
| |||||
|
|
|
|
|
|
| |||||
American depository receipts | |||||||||||
| United Kingdom | ||||||||||
Healthcare | 304,734 | 261,105 | 0.04 | ||||||||
Cayman Islands | |||||||||||
Healthcare | 103,180 | 53,101 | 0.01 | ||||||||
| |||||||||||
Total American depository receipts | 407,914 |
| 314,206 |
| 0.05 |
| |||||
|
|
|
|
| |||||||
Total securities sold short, at fair value |
|
| 102,512,585 |
| 95,151,493 |
| 15.04 |
| |||
Descriptions |
|
|
| Fair Value |
| Percentage of Net Assets |
| ||
Derivative contracts - liabilities, at fair value | |||||||||
Equity swaps | |||||||||
| United States | ||||||||
Healthcare | 7,799,422 | 1.23 | |||||||
Total derivative contracts - liabilities, at fair value |
| 7,799,422 |
| 1.23 |
| ||||
* No individual investment security or contract constitutes greater than 5 per cent. of net assets. | |||||||||
See accompanying notes to the unaudited interim consolidated financial statements.
Unaudited Interim Consolidated Statement of Operations
For the six month periods ended 30 June 2025 and 30 June 2024
(Expressed in United States Dollars)
| 1 January 2025 to 30 June 2025 (unaudited) |
| 1 January 2024 to 30 June 2024 (unaudited) | ||
|
|
|
|
| |
Investment income |
|
|
| ||
Dividends (net of withholding tax rebate of $64,343; 30 June 2024: charge of $4,258) |
|
25,018,901 |
108,997 | ||
Interest (net of withholding taxes of $nil; 30 June 2024: $nil) |
|
2,882,320 |
1,926,737 | ||
Other |
| 1,887,044 | 837,160 | ||
Total investment income |
| 29,788,265 | 2,872,894 | ||
| |||||
| |||||
Expenses | |||||
Management fees |
| 3,668,870 | 3,436,352 | ||
Interest |
| 3,625,918 | 1,679,406 | ||
Professional fees |
| 1,028,876 | 411,615 | ||
Research costs |
| 669,362 | 341,711 | ||
Administrative fees |
| 374,785 | 356,696 | ||
Audit fees |
| 232,975 | 186,995 | ||
Directors' fees |
| 151,927 | 117,976 | ||
Dividends |
| 174 | - | ||
Other expenses |
| 694,125 | 654,822 | ||
Total expenses |
| 10,447,012 |
| 7,185,573 | |
Net investment income/(loss) |
| 19,341,253 |
| (4,312,679) | |
| |||||
Realised and change in unrealised gain/(loss) on investments, derivatives and foreign currency transactions |
|
| |||
Net realised gain/(loss) on securities and foreign currency transactions |
|
|
(1,016,201) |
10,237,597 | |
Net change in unrealised gain/(loss) on securities and foreign currency translation |
|
|
(53,826,207) |
11,361,132 | |
Net realised gain/(loss) on derivative contracts |
|
| 4,193,445 | 5,155,442 | |
Net change in unrealised gain/(loss) on derivative contracts |
|
|
(9,428,650) |
63,383,452 | |
|
|
| |||
Net realised and unrealised gain/(loss) on investments, derivatives and foreign currency transactions |
|
| (60,077,613) | 90,137,623 | |
|
|
| |||
Net increase/(decrease) in net assets resulting from operations |
|
| (40,736,360) | 85,824,944 |
See accompanying notes to the unaudited interim consolidated financial statements.
Unaudited Interim Consolidated Statement of Changes in Net Assets
For the six month period ended 30 June 2025
(Expressed in United States Dollars)
| Ordinary Share Class | Non-Controlling Interest |
|
| |
Net assets, beginning of period | 606,921,161 | 25,722,524 |
|
|
|
Operations |
|
|
Net investment income/(loss) | 19,341,253 | - |
Net realised gain/(loss) on securities and foreign currency transactions | (1,016,201) | - |
Net change in unrealised gain/(loss) on securities and foreign currency translation | (53,826,207) | - |
Net realised gain/(loss) on derivative contracts | 4,193,445 | - |
Net change in unrealised gain/(loss) on derivative contracts | (9,428,650) | - |
Income/(loss) attributable to Non-Controlling Interest | 1,538,582 | (1,538,582) |
|
| |
Net change in net assets resulting from operations | (39,197,778) | (1,538,582) |
|
| |
Capital transactions |
|
|
Share buyback (Gross of $10,147 transaction costs; 30 June 2024: $22,681) (Note 9) | (6,708,388) | - |
Net change in net assets resulting from capital transactions | (6,708,388) | - |
|
|
|
Net assets, end of period | 561,014,995 | 24,183,942 |
See accompanying notes to the unaudited interim consolidated financial statements.
Unaudited Interim Consolidated Statement of Changes in Net Assets
For the six month period ended 30 June 2024
(Expressed in United States Dollars)
| Ordinary Share Class | Non-Controlling Interest |
|
| |
Net assets, beginning of period | 399,283,811 | 29,739,146 |
|
|
|
Operations |
|
|
Net investment income/(loss) | (4,312,679) | - |
Net realised gain/(loss) on securities and foreign currency transactions | 10,237,597 | - |
Net change in unrealised gain/(loss) on securities and foreign currency translation | 11,361,132 | - |
Net realised gain/(loss) on derivative contracts | 5,155,442 | - |
Net change in unrealised gain/(loss) on derivative contracts | 63,383,452 | - |
Income/(loss) attributable to Non-Controlling Interest | 843,756 | (843,756) |
|
| |
Net change in net assets resulting from operations | 86,668,700 | (843,756) |
|
| |
Capital transactions |
|
|
Issuance of Ordinary Shares (net of issuance cost of $6,473,897) | 180,781,065 | - |
Share buyback (Gross of $22,681 transaction costs; 30 June 2023: $nil) (Note 9) | (11,340,306) | - |
Net change in net assets resulting from capital transactions | 169,440,759 | - |
|
|
|
Net assets, end of period | 655,393,270 | 28,895,390 |
See accompanying notes to the unaudited interim consolidated financial statements.
Unaudited Interim Consolidated Statement of Cash Flows
For the six month periods ended 30 June 2025 and 30 June 2024
(Expressed in United States Dollars)
|
|
|
1 January 2025 to 30 June 2025 (unaudited) |
1 January 2024 to 30 June 2024 (unaudited) | ||
| Cash flows from operating activities | |||||
| Net increase/(decrease) in net assets resulting from operations | (40,736,360) | 85,824,944 | |||
| Adjustments to reconcile net change in net assets resulting from operations to net cash provided by/(used in) operating activities: | |||||
| Net realised (gain)/loss on securities and foreign currency transactions | 1,016,201 | (10,237,597) | |||
| Net change in unrealised (gain)/loss on securities and foreign currency translation |
53,826,207 |
(11,361,132) | |||
| Net realised (gain)/loss on derivative contracts | (4,193,445) | (5,155,442) | |||
| Net change in unrealised (gain)/loss on derivative contracts | 9,428,650 | (63,383,452) | |||
| Effect of exchange rate changes on cash and cash equivalents | (1,113,758) | (109,564) | |||
| Purchases of investments in securities | (199,936,589) | (350,475,670) | |||
| Proceeds from sales of investments in securities | 142,741,710 | 220,618,160 | |||
| Proceeds from securities sold short | 58,927,403 | 38,323,325 | |||
| Payments for securities sold short | (16,765,694) | (12,818,107) | |||
| Proceeds from derivative contracts | 8,701,968 | 16,122,019 | |||
| Payments for derivative contracts | (25,454,592) | (60,925,651) | |||
| Accretion of bond discount | (2,171) | (1,628) | |||
| Changes in operating assets and liabilities: | |||||
| Other assets | 33,841 | 1,655,862 | |||
| (Receivable from)/payable for unsettled trades | 4,080,685 | (6,786,057) | |||
| Due to brokers | 24,986,274 | 62,103,787 | |||
| Accrued expenses | 124,274 | (1,489,677) | |||
| Net cash provided by/(used in) operating activities | 15,664,604 | (98,095,880) | |||
| ||||||
| Cash flows from financing activities | |||||
| Net proceeds from issuance of shares * | - | 108,419,956 | |||
| Share buyback | (6,708,388) | (11,340,306) | |||
| Net cash provided by/(used in) financing activities |
| (6,708,388) | 97,079,650 | ||
| Net change in cash and cash equivalents | 8,956,216 | (1,016,230) | |||
| Cash, cash equivalents, and restricted cash, beginning of the period | 33,350,500 | 60,608,767 | |||
| Cash, cash equivalents, and restricted cash, end of the period |
| 42,306,716 |
| 59,592,537 | |
| ||||||
| At 30 June 2025, the amounts categorised in cash, cash equivalents, and restricted cash include the following: | |||||
| Cash and cash equivalents | 2,661,257 | 6,757,859 | |||
| Due from brokers | 39,645,459 | 52,834,678 | |||
| Total | 42,306,716 | 59,592,537 | |||
|
|
|
|
| ||
| Supplemental disclosure of cash flow information |
|
|
| ||
| Cash paid during the period for interest | 3,543,147 | 1,406,135 | |||
| ||||||
Cancellation of shares in RTW Biotech Opportunities Ltd received in Arix acquisition | - | 59,221,117 | ||||
| ||||||
| * In kind financing activities: | |||||
| Non-cash assets received from Arix acquisition, comprised of: | |||||
| Investments in securities | - | 129,409,264 | |||
| Derivative contracts | - | 1,799,515 | |||
| Other assets | - | 373,447 | |||
Refer to notes 1 and 9 for further details regarding the Arix acquisition.
See accompanying notes to the unaudited interim consolidated financial statements.
Notes to the Unaudited Interim Consolidated Financial Statements
For the six month period ended 30 June 2025
(Expressed in United States Dollars)
1. Nature of operations and summary of significant accounting policies
RTW Biotech Opportunities Ltd (the "Company") is a publicly listed Guernsey non-cellular company limited by shares. The Company was originally incorporated in the State of Delaware, United States of America, and re-domiciled into Guernsey under the Companies Law on 2 October 2019 with registration number 66847 on the Guernsey Register of Companies. On 30 October 2019, all of the issued Ordinary Shares of the Company were listed and admitted to trading on the Specialist Fund Segment of the London Stock Exchange under the ticker symbol: RTW. Subsequently, on 6 August 2021, the Company's Ordinary Shares were admitted to trading on the Premium Segment of the London Stock Exchange (the former standard and premium listing segments of the London Stock Exchange Main Market were consolidated into a single segment on 29 July 2024) with the additional ticker symbol: RTWG denoting the Sterling price. The RTWG ticker was consolidated into the USD line effective October 2024 and the Company ceased trading the GBP quote. The original ticker, RTW, continues to denote the US Dollar price.
In 2022, the Company transferred its right to the profits and losses attributable to the Group's portfolio of assets to its wholly owned subsidiary, RTW Biotech Opportunities Operating Ltd (the "Subsidiary"). All the income and expenses of the Subsidiary are consolidated with the income and expenses of the Group.
On 13 February 2024, the Group completed the acquisition of the assets of Arix Bioscience plc. To facilitate the acquisition, the Subsidiary formed RTW Biotech UK Limited (the "UK Subsidiary") as a wholly owned subsidiary of the Subsidiary to manage and integrate the Arix Bioscience plc acquired entities and assets, based on the regulatory and operational landscape in the UK. The transaction was announced on 1 November 2023 and was effected through a scheme of reconstruction and the voluntary winding‐up of Arix under section 110 of the Insolvency Act 1986. The details around this transaction are further disclosed within the unaudited interim consolidated statement of cash flows and within Note 9.
In April 2025, the Subsidiary formed RTW Biotech ALI LLC (the "SPV"), a Delaware limited liability company, to serve as an intermediate blocker to manage tax exposure through which the Subsidiary holds an investment. The Subsidiary is the sole member of the SPV. All the income and gains/losses will be allocated or distributed to the SPV. All the income and expenses of the SPV are consolidated with the income and expenses of the Company.
The Group seeks to use equity capital (from the net proceeds of any share issuance or, where appropriate, from the net proceeds of investment divestments or other related profits) to provide seed and additional growth capital to the private investments. To mitigate cash-drag, the uninvested portion is invested across public stocks largely replicating the public stock portfolios of RTW's existing US-based funds. The Group focuses on creating, building, and supporting world-class life sciences, biopharmaceutical and medical technology companies. The Group's investment objective is to generate attractive risk-adjusted returns through investments in securities, both equity and debt, long and short, of companies with a focus on the pharmaceutical sector.
Pursuant to an investment management agreement, the Group is managed by RTW Investments, LP, a Delaware limited partnership, to provide the Group with discretionary portfolio management, risk management services and certain other services. The Investment Manager is an investment adviser registered with the U.S. Securities and Exchange Commission under the Investment Advisers Act of 1940.
Basis of presentation
The unaudited interim consolidated financial statements are expressed in United States Dollars. The unaudited interim consolidated financial statements which give a true and fair view and have been prepared in accordance with US generally accepted accounting principles ("US GAAP") and are in compliance with the Companies (Guernsey) Law, 2008. The entities comprised within the Group are investment companies and follow the accounting and reporting guidance in Financial Accounting Standards Board's ("FASB") Accounting Standards Codification Topic 946, Financial Services - Investment Companies.
The Directors consider that it is appropriate to adopt a going concern basis of accounting in preparing the unaudited interim consolidated financial statements. In reaching this assessment, the Directors have considered a wide range of information relating to present and future conditions including the balance sheets, future projections, cash flows and the longer-term strategy of the business.
Principles of consolidation
The unaudited interim consolidated financial statements include the accounts of the Company consolidated with the accounts of the Subsidiary, the UK Subsidiary, and the SPV. All inter-group balances have been eliminated upon consolidation. The Subsidiary is incorporated in Guernsey, the UK Subsidiary is incorporated in the United Kingdom, and the SPV is incorporated in Delaware.
Non-Controlling Interest
An affiliate of the Investment Manager, RTW Venture Performance LLC, holds an interest in the Subsidiary. The Non-Controlling Interest captures both Performance Allocation and mark to market movements on the New Performance Allocation Share held by RTW Venture Performance LLC in the Subsidiary. For the period ended 30 June 2025, $1,538,582 of the loss attributable to the Non-Controlling Interest was comprised of mark to market movements of Notional Ordinary Shares (31 December 2024: $1,259,780), with $nil of the loss related to a reversal of uncrystallized performance allocation from Ordinary Shareholders to the Performance Allocation Share Class (31 December 2024: $2,756,842).
Cash, cash equivalents, and restricted cash
Cash represents cash deposits held at financial institutions. Cash equivalents include short-term highly liquid investments of sufficient credit quality that are readily convertible to known amounts of cash and have original maturities of three months or less. Cash equivalents are carried at cost plus accrued interest, which approximates fair value. Cash equivalents are held for the purpose of meeting short-term liquidity requirements, rather than for investment purposes. As at 30 June 2025 and 31 December 2024, the Group had no cash equivalents.
Restricted cash is subject to a legal or contractual restriction by third parties as well as a restriction as to withdrawal or use, including restrictions that require the funds to be used for a specified purpose and restrictions that limit the purpose for which the funds can be used. The Group considers cash pledged as collateral for securities sold short, cash collateral posted with counterparties for derivative contracts and further amounts due from brokers to be restricted cash, as outlined in Note 3.
Fair value - definition and hierarchy
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (i.e. the 'exit price') in an orderly transaction between market participants at the measurement date.
In determining fair value, the Group uses various valuation techniques. A fair value hierarchy for inputs is used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs are to be used when available. Observable inputs are those that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Group.
Unobservable inputs reflect the Group's assumptions about the inputs market participants would use in pricing the asset or liability based on the best information available in the circumstances. The fair value hierarchy is categorised into three levels based on the inputs as follows:
Level 1 - Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities that the Group has the ability to access. Valuation adjustments are not applied to Level 1 investments. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these investments does not entail a significant degree of judgement.
Level 2 - Valuations based on inputs, other than quoted prices included in Level 1, that are observable, either directly or indirectly.
Level 3 - Valuations based on inputs that are unobservable and significant to the overall fair value measurement.
Investments in private investment companies measured using net asset value as a practical expedient are not categorised in the fair value hierarchy.
The availability of valuation techniques and observable inputs can vary from investment to investment and is affected by a wide variety of factors, including the type of investment, whether the investment is new and not yet established in the marketplace, and other characteristics particular to the transaction. To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgement. Those estimated values do not necessarily represent the amounts that may be ultimately realised due to the occurrence of future circumstances that cannot be reasonably determined. Because of the inherent uncertainty of valuation, those estimated values may be materially higher or lower than the values that would have been used had a ready market for the investments existed. Accordingly, the degree of judgement exercised by the Group in determining fair value is greatest for investments categorised in Level 3. In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, for disclosure purposes, the level in the fair value hierarchy within which the fair value measurement falls in its entirety is determined based on the lowest level input that is significant to the fair value measurement.
Fair value is a market-based measure considered from the perspective of a market participant rather than an entity-specific measure. Therefore, even when market assumptions are not readily available, the Group's own assumptions are set to reflect those that market participants would use in pricing the asset or liability at the measurement date. The Group uses prices and inputs that are current as of the measurement date, including periods of market dislocation. In periods of market dislocation, the observability of prices and inputs may be reduced for many investments. This condition could cause an investment to be reclassified to a lower level within the fair value hierarchy.
Fair value - valuation techniques and inputs
Investments in securities and securities sold short
Listed investments
The Group values investments in securities including exchange traded funds and securities sold short that are freely tradable and are listed on a national securities exchange or reported on the NASDAQ national market at their closing sales price as of the valuation date. To the extent these securities are actively traded and valuation adjustments are not applied, they are categorised in Level 1 of the fair value hierarchy. Securities traded on inactive markets or valued by reference to similar instruments or where a discount may be applied are categorised in Level 2 or 3 of the fair value hierarchy.
Unlisted investments
Unlisted investments are valued at fair value by the Directors following a detailed review and appropriate challenge of the valuations proposed by the Investment Manager. As part of their valuation process, the Investment Manager engages Independent Valuers to challenge their assessed fair value on certain unlisted investments. The Investment Manager's unlisted investment valuation policy applies techniques consistent with the IPEV Guidelines.
The valuation techniques applied are either a market-based approach, an income approach such as discounted cash flows, or where available, a net asset value practical expedient approach. A combination of the valuation techniques mentioned may also be utilised. The IPEV Guidelines recognise that the price of a recent transaction, if resulting from an orderly transaction, generally represents fair value as at the transaction date and may be an appropriate starting point for estimating fair value at subsequent measurement dates. Consideration is given to the facts and circumstances as at the subsequent measurement date including changes in the market and/or performance of the investee company. Milestone analysis is used where appropriate to incorporate operational progress at the investee company level. In addition, a trigger event such as a subsequent round of financing by the investee company would influence the market technique used to calibrate fair value at the measurement date. Where appropriate, a probability-weighted expected return method ("PWERM") may be employed when different potential outcomes (e.g. IPO, round of financing, stay private, dissolution, etc.) are utilised to derive the value of investments held.
The market approach utilises guideline public companies relying on projected revenues and/or earnings metrics to derive an indicative enterprise value. Due to the nature of the investments, being in the early stages of development, the projected revenues are typically used as a proxy for stable state revenue. A selected multiple is then applied based on the observed market multiples of the guideline public companies. To reflect the risk associated with the achievement of the projected financial metrics and the early development stage of each of the investments, the indicative enterprise value is discounted at an appropriate rate.
The income approach utilises the discounted cash flow method. Projected cash flows for each investment are discounted to determine the enterprise value.
Where applicable, the indicative enterprise value has been determined using a back-solve model based on the pricing of the most recent round of financing. The internal rate of return for each investment is compared to the selected venture capital rate applied in the market approach to assess the reasonableness of the indicated value implied by each financing round. The derived enterprise value is allocated to the equity class on either a fully diluted basis or using an option pricing model. The resulting indicative value on a per share basis is then multiplied by the number of shares to derive the fair market value.
American depository receipts
The Group values investments in American depositary receipts that are freely tradable and are listed on a national securities exchange or reported on the NASDAQ national market at their last reported sales price as of the valuation date. These investments are categorised in Level 1 of the fair value hierarchy.
Convertible notes
The Group values investments in convertible notes in accordance with the unlisted investments section above. As of 30 June 2025, these investments are all categorised in Level 3 of the fair value hierarchy.
Convertible preferred stock
The Group values Level 1 investments in convertible preferred stock that are listed on a national securities exchange at their closing sales price as of the valuation date. Level 2 investments in convertible preferred stock are valued with certain adjustments to the underlying public stocks closing sales price that is listed on a national securities exchange. Level 3 investments in convertible preferred stock are valued in accordance with the unlisted investments section above. As of 30 June 2025, these investments are categorised in Level 3 of the fair value hierarchy.
Corporate bonds
The fair value of corporate bonds is estimated using recently executed transactions, market price quotations (where observable), bond spreads, or credit default swap spreads. The spread data used is for the same maturity as the bond. If the spread data does not reference the issuer, then data that references a comparable issuer is used. When observable price quotations are not available, fair value is determined based on cash flow models using yield curves, bond or single name credit default swap spreads, and recovery rates based on collateral values as key inputs. As of 30 June 2025, these investments are categorised in Level 3 of the fair value hierarchy.
Investment in private investment companies
The Group values investment in private investment companies using the net asset values provided by the underlying private investment companies as a practical expedient. The Group applies the practical expedient to its private investment companies on an investment-by-investment basis and consistently with the Group's entire position in a particular investment, unless it is probable that the Group will sell a portion of an investment at an amount different from the net asset value of the investment.
Private investment in public equity
Private investment in public equity ("PIPE") cannot be offered for sale to the public until the issuer complies with certain statutory or contractual requirements. Such securities traded on inactive markets or valued by reference to similar instruments or where a discount may be applied are generally categorised in Level 2. However, to the extent that significant inputs used to determine liquidity discounts are unobservable, PIPE may be categorized in Level 3 of the fair value hierarchy. As of 30 June 2025, these investments are categorised in Level 2 of the fair value hierarchy and are recognised as warrants within the Schedule of Investments.
Revenue Based Financing Agreement
These represent structured, non-dilutive financing alternatives for businesses seeking to raise capital in lieu typically of issuing equity. The Group may enter into a contract with an undertaking that owns the revenue interest in one or more healthcare products and such undertaking also typically plays the principal role in commercialization, marketing and sales of such product or products. This contract entitles the Group to receive a share of revenue from a stream of cash flow payments based on the sales of such product or products.
The valuation is based on an income approach utilizing management's internal projections or sell-side equity research analysts' consensus estimates in the absence of adequate brokerage analyst coverage. The projections take into account contractual terms specific to each revenue based financing investment and are present valued based on a discount rate based on the prime rate adjusted for additional investment-specific risk that aligns to the debt-like nature of the projected cash flows specific to the Group. As of 30 June 2025, these investments are categorised in Level 3 of the fair value hierarchy.
Derivative contracts
Equity swaps
Equity swaps may be centrally cleared or traded on the over-the-counter market. The fair value of equity swaps is calculated based on the terms of the contract and current market data, such as changes in fair value of the reference asset. The fair value of equity swaps is generally categorised in Level 2 of the fair value hierarchy.
Warrants
Warrants that are listed on major securities exchanges are valued at their last reported sales price as of the valuation date. The fair value of over-the-counter ("OTC") warrants is determined using the Black-Scholes option pricing model, a valuation technique that follows the income approach. This pricing model takes into account the contract terms (including maturity) as well as multiple inputs, including time value, implied volatility, equity prices, interest rates and currency rates. Warrants are categorised in all levels of the fair value hierarchy.
Contingent value rights
Contingent value rights that are not traded on an organized facility are valued using a market approach or such other analysis and information as the Group may determine. As of 30 June 2025, these investments are categorised in Level 3 of the fair value hierarchy.
Fair value - valuation processes
The Group establishes valuation processes and procedures to ensure that the valuation techniques are fair and consistent, and valuation inputs are supportable. The Group designates the Investment Manager's Valuation Committee to oversee the entire valuation process of the Group's investments. The Valuation Committee comprises various members of the Investment Manager, including those separate from the Group's portfolio management and trading functions, and reports to the Board.
The Valuation Committee is responsible for developing the Group's written valuation processes and procedures, conducting periodic reviews of the valuation policies, and evaluating the overall fairness and consistent application of the valuation policies.
The Investment Manager's Valuation Committee meets on a monthly basis or more frequently, as needed, to determine the valuations of the Group's Level 3 investments. Valuations determined by the Valuation Committee are required to be supported by market data, third-party pricing sources, industry-accepted pricing models, counterparty prices or other methods they deem to be appropriate, including the use of internal proprietary pricing models.
The Group periodically tests its valuations of Level 3 investments by performing back-testing. Back-testing involves the comparison of sales proceeds of those investments to the most recent fair values reported and, if necessary, uses the findings to recalibrate its valuation procedures.
On a regular basis, the Group engages the services of third-party valuation firms, the Independent Valuers, to perform an independent review of the valuation of the Group's Level 3 investments and the Group may adjust its valuations based on the recommendations from the Investment Manager's Valuation Committee.
Translation of foreign currency
Assets and liabilities denominated in foreign currencies are translated into United States Dollar amounts at the year end exchange rates. Transactions denominated in foreign currencies, including purchases and sales of investments, and income and expenses, are translated into United States Dollar amounts on the transaction date. Adjustments arising from foreign currency transactions are reflected in the unaudited interim consolidated statement of operations.
The Group does not isolate that portion of the results of operations arising from the effect of changes in foreign exchange rates on investments from fluctuations arising from changes in market prices of investments held. Such fluctuations are included in net realised and change in unrealised gain/(loss) on securities, derivatives and foreign currency transactions in the consolidated statement of operations.
Reported net realised gain/(loss) from foreign currency transactions arise from sales of foreign currencies; currency gains or losses realised between the trade and settlement dates on securities transactions; and the difference between the amounts of dividends, interest, and foreign withholding taxes recorded on the Group's books and the United States Dollar equivalent of the amounts actually received or paid.
Net change in unrealised gain/(loss) from foreign currency translation of assets and liabilities arises from changes in the fair values of assets and liabilities, other than investments in securities at the end of the period, resulting from changes in exchange rates.
Investment transactions and related investment income
Investment transactions are accounted for on a trade date basis. Realised gains and losses on investment transactions have been calculated on a specific identification method.
Dividends are recorded on the ex-dividend date and interest is recognised on the accrual basis.
Withholding taxes on foreign dividends have been provided for in accordance with the Group's understanding of the applicable country's rules and rates.
Offsetting of amounts related to certain contracts
Amounts due from and to brokers are presented on a net basis, by counterparty, to the extent the Group has the legal right to offset the recognised amounts and intends to settle on a net basis.
The Group has elected not to offset fair value amounts recognised for cash collateral receivables and payables against fair value amounts recognised for derivative positions executed with the same counterparty under the same master netting arrangement. At 30 June 2025, the Group had cash collateral receivables of $39,415,944 (31 December 2024: $23,390,565) (see Note 3) with derivative counterparties under the same master netting arrangement.
Income taxes
The Company and Subsidiary are exempt from taxation in Guernsey and were each charged an annual exemption fee of GBP 1,600 (2024: GBP 1,600). The Group will only be liable to tax in Guernsey in respect of income arising or accruing from a Guernsey source, other than from a relevant bank deposit. It is not anticipated that such Guernsey source taxable income will arise. The Group is managed so as not to be resident in the UK for UK tax purposes.
The Group recognises tax benefits of uncertain tax positions only where the position is more likely than not to be sustained assuming examination by a tax authority based on the technical merits of the position. In evaluating whether a tax position has met the recognition threshold, the Group must presume the position will be examined by the appropriate taxing authority and that taxing authority has full knowledge of all relevant information. A tax position meeting the more likely than not recognition threshold is measured to determine the amount of benefit to recognise in the Group's unaudited interim consolidated financial statements. Income tax and related interest and penalties would be recognised as a tax expense in the unaudited interim consolidated statement of operations if the tax position was deemed to meet the more likely than not threshold.
The Investment Manager has analysed the Group's tax positions and has concluded no liability for unrecognised tax benefits should be recorded related to uncertain tax positions. Further, management is not aware of any tax positions for which it is reasonably possible the total amounts of unrecognised tax benefits will significantly change in the next twelve months.
The Company, UK Subsidiary, Subsidiary, and SPV each file income tax returns in the US federal jurisdiction and, as applicable, in US state or local jurisdictions, or non-US jurisdictions. Generally, the Group was subject to income tax examinations by major taxing authorities for each tax period since inception. Based on its analysis, the Group determined that it had not incurred any liability for unrecognised tax benefits as of 30 June 2025 or 31 December 2024.
Use of estimates
Preparing unaudited interim consolidated financial statements in accordance with US GAAP requires management to make estimates and assumptions in determining the reported amounts of assets and liabilities, including the fair value of investments, and disclosure of contingent assets and liabilities as of the date of the unaudited interim consolidated financial statements and the reported amounts of income and expenses during the reporting period. Actual results could differ from those estimates.
2. Fair value measurements
The Group's assets and liabilities recorded at fair value have been categorised based upon a fair value hierarchy as described in the Group's significant accounting policies in Note 1.
The following table presents information about the Group's assets and liabilities measured at fair value as of 30 June 2025:
Level 1 | Level 2 | Level 3 | Investments measured at net asset value* | Total | ||
| ||||||
Assets (at fair value) | ||||||
Investments in securities | ||||||
Common stocks | 320,947,683 | 109,967 | 9,035,930 | - | 330,093,580 | |
Convertible preferred stocks | - | - | 132,392,027 | - | 132,392,027 | |
American depository receipts | 66,943,228 | - | - | - | 66,943,228 | |
Convertible notes | - | - | 56,230,080 | - | 56,230,080 | |
Investment in private investment companies |
- |
- |
- |
17,624,249 |
17,624,249 | |
Revenue based financing agreement |
- |
- |
186,910 |
- |
186,910 | |
Corporate bonds | - | - | - | - | - | |
Total investments in securities | 387,890,911 | 109,967 | 197,844,947 | 17,624,249 | 603,470,074 | |
Derivative contracts | ||||||
Warrants | 308 | 94,938,244 | 20,692 | - | 94,959,244 | |
Equity swaps | - | 22,113,533 | - | - | 22,113,533 | |
Contingent value rights | - | - | 1,697,725 | - | 1,697,725 | |
Total derivative contracts | 308 | 117,051,777 | 1,718,417 | - | 118,770,502 | |
387,891,219 |
117,161,744 |
199,563,364 |
17,624,249 |
722,240,576 | ||
Liabilities (at fair value) | ||||||
Securities sold short | ||||||
Common stocks | 115,696,794 | - | - | - | 115,696,794 | |
American depository receipts | 10,606,986 | - | - | - | 10,606,986 | |
Total securities sold short | 126,303,780 | - | - | - | 126,303,780 | |
Derivative contracts | ||||||
Equity swaps | - | 4,875,333 | - | - | 4,875,333 | |
Total derivative contracts | - | 4,875,333 | - | - | 4,875,333 | |
126,303,780 |
4,875,333 |
- |
- |
131,179,113 | ||
* The Group's investment in private investment companies that are valued at their net asset value are not categorised within the fair value hierarchy.
The following table presents information about the Group's assets and liabilities measured at fair value as of 31 December 2024:
Level 1 | Level 2 | Level 3 | Investments measured at net asset value* | Total | ||
| ||||||
Assets (at fair value) | ||||||
Investments in securities | ||||||
Common stocks | 362,223,884 | 266,171 | 1,457,470 | - | 363,947,525 | |
Convertible preferred stocks | - | - | 164,325,035 | - | 164,325,035 | |
Convertible notes | - | - | 32,744,748 | - | 32,744,748 | |
American depository receipts | 30,724,375 | - | - | - | 30,724,375 | |
Investment in private investment companies |
- |
- |
- |
19,094,800 |
19,094,800 | |
Revenue based financing agreement |
- |
- |
174,613 |
- |
174,613 | |
Corporate bonds | - | - | - | - | - | |
Total investments in securities | 392,948,259 | 266,171 | 198,701,866 | 19,094,800 |
611,011,096 | |
Derivative contracts | ||||||
Warrants | 367 | 87,127,278 | 919,671 | - | 88,047,316 | |
Equity swaps | - | 20,526,482 | - | - | 20,526,482 | |
Contingent value rights | - | - | 1,603,374 | - | 1,603,374 | |
Total derivative contracts | 367 | 107,653,760 | 2,523,045 | - | 110,177,172 | |
392,948,626 |
107,919,931 |
201,224,911 |
19,094,800 |
721,188,268 | ||
Liabilities (at fair value) | ||||||
Securities sold short | ||||||
Common stocks | 94,837,287 | - | - | - | 94,837,287 | |
American depository receipts | 314,206 | - | - | - | 314,206 | |
Total securities sold short | 95,151,493 | - | - | - | 95,151,493 | |
Derivative contracts | ||||||
Equity swaps | - | 7,799,422 | - | - | 7,799,422 | |
Total derivative contracts | - | 7,799,422 | - | - | 7,799,422 | |
95,151,493 |
7,799,422 |
- |
- |
102,950,915 | ||
* The Group's investment in private investment companies that are valued at their net asset value are not categorised within the fair value hierarchy.
The following tables summarise the valuation techniques and significant unobservable inputs used for the Group's investments that are categorised within Level 3 of the fair value hierarchy as of 30 June 2025 and 31 December 2024:
Fair value at 30 June 2025 | Valuation techniques | Significant unobservable inputs | Range of inputs |
| ||||
Assets (at fair value) |
| |||||||
Investments in securities |
| |||||||
| Convertible preferred stocks | 72,030,588 | Recent transaction price | n/a | n/a |
| ||
41,189,623 | Probability-weighted expected return method ("PWERM") | WACC Revenue multiples Market step-up multiple
| 30% 4.0x 0.5x - 1.3x
| |||||
Market rate of returns | (20%) - 0% | |||||||
19,162,793 | Discounted cash flow | WACC | 15% - 31% | |||||
and/or market approach | Revenue multiples | 3.3x - 4.0x | ||||||
Market rate of returns
| (13%) - 10% | |||||||
9,023 | Liquidation value | n/a | n/a | |||||
Convertible notes | 48,213,825 | PWERM | Discount rate | 6% - 9% |
| |||
Market step-up multiple | 0.5x - 0.7x |
| ||||||
Market rate of returns | 0% |
| ||||||
Expected volatility | 60% |
| ||||||
8,016,255 | Recent transaction price | Interest rate | 7% - 12% | |||||
Common stocks | 8,501,327 | Market approach | EBITDA multiples | 3.0x - 6.5x |
| |||
Revenue multiples | 0.5x - 1.7x |
| ||||||
Market rate of returns
| (30%) |
| ||||||
372,115 | Probability-weighted expected return method ("PWERM") | Market step-up multiple
| 0.5x - 0.7x |
| ||||
162,488 | Liquidation value | n/a | n/a |
| ||||
Revenue interest financing | 186,910 | Discounted cash flow and/or market approach | WACC | 27%
|
| |||
Total investments in securities | 197,844,947 |
|
|
|
| |||
|
|
|
|
|
| |||
Derivative contracts |
|
|
|
|
| |||
Contingent value rights | 1,697,725 | Recent transaction price | n/a | n/a |
| |||
Warrants | 20,692 | Option pricing model | Expected volatility | 40% |
| |||
Total derivative contracts | 1,718,417 |
| ||||||
Fair value at 31 December 2024 | Valuation techniques | Significant unobservable inputs | Range of inputs |
| |||||
Assets (at fair value) |
| ||||||||
Investments in securities |
| ||||||||
| Convertible preferred stocks | 56,837,402 | Recent transaction price | n/a | n/a |
| |||
37,870,153 | Discounted cash flow | WACC | 10% - 31% |
| |||||
and/or market approach | Revenue multiples | 2.0x - 4.0x |
| ||||||
Market rate of returns
| (13%) - 15% |
| |||||||
69,559,998 | Probability-weighted expected return method ("PWERM") | WACC Revenue multiples Market step-up multiple | 10% - 20% 4.0x 0.8x - 2.1x | ||||||
Market rate of returns | (5%) - 5% | ||||||||
57,482 | Liquidation value | n/a | n/a | ||||||
Convertible notes | 32,156,487 | PWERM | Discount rate | 6% -12% | |||||
Market step-up multiple | 0.9x - 1.2x |
| |||||||
Market rate of returns | (5%) - 5% |
| |||||||
Expected volatility | 60% |
| |||||||
588,261 | Recent transaction price | n/a | n/a |
| |||||
Common stocks | 246,828 | Recent transaction price | n/a | n/a |
| ||||
375,605 | Liquidation value | n/a | n/a | ||||||
835,037 | Probability-weighted expected return method ("PWERM") | Market step-up multiple Market Rate of Returns
| 0.9x - 1.2x 5% - 5%
|
| |||||
Revenue interest financing | 174,613 | Discounted cash flow and/or market approach | WACC | 28% - 28%
|
| ||||
Total investments in securities | 198,701,866 |
|
|
|
| ||||
|
|
|
|
|
| ||||
Derivative contracts | |||||||||
Contingent value rights | 1,603,374 | Recent transaction price | n/a | n/a | |||||
Warrants | 919,671 | Discounted cash flow and/or market approach and option pricing model | Expected volatility | 40% | |||||
Total derivative contracts | 2,523,045 | ||||||||
The significant unobservable inputs used in the fair value measurements of Level 3 common stock, convertible preferred stocks, convertible notes, and warrants include, but are not limited to, WACC, revenue and/or earnings multiple, market rate of return, and expected volatility. Increases in the WACC in isolation would result in a lower fair value for the security, and vice versa. Increases in multiples and/or market rate of returns in isolation would result in a higher fair value of the security, and vice versa. A change in volatility in isolation could result in a higher or lower fair value for the security.
The below table presents additional information about Level 3 assets and liabilities measured at fair value. Both observable and unobservable inputs may be used to determine the fair value of positions that the Group has classified within the Level 3 category. As a result, the unrealised gains and losses for assets and liabilities within the Level 3 category may include changes in fair value that were attributable to both observable and unobservable inputs.
Changes in Level 3 assets and liabilities measured at fair value for the period ended 30 June 2025 were as follows:
Balance beginning 1 January 2025 (unaudited) | Realised gains/ (losses)(a) (unaudited) | Change in Unrealised gains/ (losses)(a) (unaudited) | Purchases (unaudited) | Sales (unaudited) | Transfers into/(from) Level 3(b) (unaudited) | Ending balance 30 June 2025 (unaudited) | ||
Assets (at fair value) | ||||||||
Investments in securities | ||||||||
Common stocks | 1,457,470 | (2,084,260) | 2,762,723 | 6,899,997 | - | - | 9,035,930 | |
Convertible preferred stocks |
164,325,035 |
- |
(39,204,397) |
14,697,022 |
- |
(7,425,633) |
132,392,027 | |
Convertible notes | 32,744,748 | - | (952,286) | 28,501,294 | - | (4,063,676) | 56,230,080 | |
Revenue based financing agreement |
174,613 |
- |
12,297 |
- |
- |
- |
186,910 | |
Total investments in securities |
198,701,866 |
(2,084,260) |
(37,381,663) |
50,098,313 |
- |
(11,489,309) |
197,844,947 | |
Derivative contracts | ||||||||
Warrants | 919,671 | - | (272,312) | - | - | (626,667) | 20,692 | |
Contingent value rights |
1,603,374 |
- |
94,351 |
- |
- |
- |
1,697,725 | |
Total derivative contracts |
2,523,045 |
- |
(177,961) |
- |
- |
(626,667) |
1,718,417 | |
Changes in Level 3 assets and liabilities measured at fair value for the year ended 31 December 2024 were as follows:
Balance beginning 1 January 2024 (audited) | Realised gains/ (losses)(a) (audited) | Change in Unrealised gains/ (losses)(a) (audited) | Purchases (audited) | Sales (audited) | Transfers into/(from) Level 3(c) (audited) | Ending balance 31 December 2024 (audited) | |||||||||
Assets (at fair value) |
| ||||||||||||||
Investments in securities | |||||||||||||||
Common stocks | 904,339 | 3,423,828 | (8,477,436) | 9,030,018 | (4,897,750) | 1,474,471 | 1,457,470 | ||||||||
Convertible preferred stocks |
73,189,264 |
- |
32,032,300 |
67,196,769 |
- |
(8,093,298) |
164,325,035 | ||||||||
Convertible notes | 7,983,390 | 83,537 | (570,999) | 27,016,689 | (1,768,682) | 813 | 32,744,748 | ||||||||
Revenue based financing agreement |
- |
- |
13,882 |
160,731 |
- |
- |
174,613 | ||||||||
Total investments in securities |
82,076,993 |
3,507,365 |
22,997,747 |
103,404,207 |
(6,666,432) |
(6,618,014) |
198,701,866 | ||||||||
Derivative contracts | |||||||||||||||
Warrants | 697,472 | - | 221,386 | - | - | 813 | 919,671 | ||||||||
Contingent value rights |
541,706 |
812,225 |
430,401 |
466,419 |
(812,225) |
164,848 |
1,603,374 | ||||||||
Total derivative contracts |
1,239,178 |
812,225 |
651,787 |
466,419 |
(812,225) |
165,661 |
2,523,045 | ||||||||
(a) Realised and unrealised gains and losses are included in net realised and change in unrealised gain/(loss) on investments, derivatives and foreign currency transactions in the unaudited interim consolidated statement of operations.
(b) Conversions of convertible preferred stock, convertible notes and warrants into common stock.
(c) Includes conversions of preferred stock into common stock.
Changes in Level 3 unrealised gains and losses during the period for assets still held at period end were as follows:
| 30 June 2025 (unaudited) | 31 December 2024 (audited) | ||
Common stocks | (83,822) | (8,477,436) | ||
Convertible notes | (150,741) | (570,999) | ||
Convertible preferred stocks | (39,504,173) | 32,081,173 | ||
Revenue Based Financing Agreement | 12,297 | 13,882 | ||
Contingent value rights | 94,351 | 430,401 | ||
Warrants | 441 | 221,386 | ||
Change in unrealised gains and losses during the period for assets still held at period end | (39,631,647) | 23,698,407 | ||
Total realised gains and losses and unrealised gains and losses in the Group's investment in securities, derivative contracts and securities sold short are made up of the following gain and loss elements:
| 30 June 2025 (unaudited) | 31 December 2024 (audited) | ||
Realised gains | 44,711,130 | 96,931,839 | ||
Realised losses | (41,533,886) | (60,671,005) | ||
Net realised gain on securities, derivative contracts and securities sold short | 3,177,244 | 36,260,834 | ||
| 30 June 2025 (unaudited) | 31 December 2024 (audited) | ||
Change in unrealised gains | 89,952,235 | 190,826,387 | ||
Change in unrealised losses | (153,207,092) | (184,163,957) | ||
Net change in unrealised gain/(loss) on securities, derivative contracts and securities sold short | (63,254,857) | 6,662,430 | ||
As at 30 June 2025, the Group had commitments (subject to completion of certain parameters) to certain investments totalling $64,519,029 (31 December 2024: $22,390,694), which was mainly comprised of a $28,866,620 commitment to Corxel (31 December 2024: $14,651,294 commitment to the 4010 Royalty Fund).
3. Due to/from brokers
Due to/from brokers includes cash balances held with brokers and collateral on derivative transactions. Amounts due from brokers may be restricted to the extent that they serve as deposits for securities sold short or cash posted as collateral for derivative contracts.
As at 30 June 2025, due from brokers totalled $39,645,459 (31 December 2024: $27,990,478). Included within due from brokers is $229,515 (31 December 2024: $4,599,913) which can be used for investment. The Group pledged cash collateral to counterparties to over-the-counter derivative contracts of $39,415,944 (31 December 2024: $23,390,565) which is included in due from brokers.
In the normal course of business, substantially all of the Group's securities transactions, money balances, and security positions are transacted with the Group's prime brokers and counterparties, Goldman Sachs & Co. LLC, Cowen Financial Products, LLC, UBS AG, Bank of America Merrill Lynch, Morgan Stanley & Co. LLC, Jefferies & Co. and J.P. Morgan Securities, LLC. The Group is subject to credit risk to the extent any broker with which it conducts business is unable to fulfil contractual obligations on its behalf. The Group's management monitors the financial condition of such brokers and does not anticipate any losses from these counterparties.
4. Derivative contracts
In the normal course of business, the Group utilises derivative contracts in connection with its proprietary trading activities. Investments in derivative contracts are subject to additional risks that can result in a loss of all or part of an investment. The Group's derivative activities and exposure to derivative contracts are classified by the primary underlying risk, equity price risk and foreign currency exchange rate risk. In addition to its primary underlying risk, the Group is also subject to additional counterparty risk due to the inability of its counterparties to meet the terms of their contracts.
Warrants
The Group may receive warrants from its portfolio companies upon an investment in the debt or equity of a portfolio company. The warrants provide the Group with exposure and potential gains upon equity appreciation of the portfolio company's share price.
The value of a warrant has two components: time value and intrinsic value. A warrant has a limited life and expires on a certain date. As time to the expiration date of a warrant approaches, the time value of a warrant will decline. In addition, if the stock underlying the warrant declines in price, the intrinsic value of an "in the money" warrant will decline. Further, if the price of the stock underlying the warrant does not exceed the strike price of the warrant on the expiration date, the warrant will expire worthless. As a result, there is the potential for the Group to lose its entire investment in a warrant.
The Group is exposed to counterparty risk from the potential failure of an issuer of warrants to settle its exercised warrants. The maximum risk of loss from counterparty risk to the Group is the fair value of the contracts and the purchase price of the warrants. The Group considers the effects of counterparty risk when determining the fair value of its investments in warrants.
Equity swap contracts
The Group is subject to equity price risk in the normal course of pursuing its investment objectives. The Group may enter into equity swap contracts either to manage its exposure to the market or certain sectors of the market, or to create exposure to certain equities to which it is otherwise not exposed.
Equity swap contracts involve the exchange by the Group and a counterparty of their respective commitments to pay or receive a net amount based on the change in the fair value of a particular security or index and a specified notional amount.
Contingent value rights
The Group may receive contingent value rights during mergers, acquisitions, or divestitures. Contingent value rights are designed to provide the Group with additional compensation or benefits contingent upon the occurrence of specific future events, such as regulatory approvals, milestones related to product development or commercialization, or the achievement of certain financial targets. Contingent value rights are subject to the uncertainty of payout, as their value hinges on the occurrence of specific events. The Group considers the uncertainty when determining the fair value of its investments in contingent value rights.
Volume of derivative activities
The Group considers the average month-end notional amounts during the period, categorised by primary underlying risk, to be representative of the volume of its derivative activities during the period ended 30 June 2025:
30 June 2025 (unaudited) |
| 31 December 2024 (audited) | |||||||
Long exposure |
| Short exposure |
| Long exposure |
| Short exposure | |||
Primary underlying risk | Notional amounts | Notional amounts |
| Notional amounts |
| Notional amounts | |||
Equity price | |||||||||
Warrants(a) | 97,082,186 | - | 60,394,443 | 30,266,515 | |||||
Equity swaps | 63,468,850 | 41,794,079 | 92,282,619 | - | |||||
Contingent value rights | 2,031,649 | - | 2,070,315 | - | |||||
162,582,685 |
| 41,794,079 |
| 154,747,377 |
| 30,266,515 | |||
(a) Notional amounts presented for warrants are based on the fair value of the underlying shares as if the warrants were exercised at each respective month end date.
Impact of derivatives on the unaudited interim consolidated statement of assets and liabilities and unaudited interim consolidated statement of operations
The following tables identify the fair value amounts of derivative instruments included in the unaudited interim consolidated statement of assets and liabilities as derivative contracts, categorised by primary underlying risk, at 30 June 2025 and 31 December 2024. The following table also identifies the gain and loss amounts included in the unaudited interim consolidated statement of operations as net realised gain/(loss) on derivative contracts and net change in unrealised gain/(loss) on derivative contracts, categorised by primary underlying risk, for the periods ended 30 June 2025 and 30 June 2024.
30 June 2025 (unaudited) | |||||||||
Primary underlying risk | Derivative assets |
| Derivative liabilities | Realised gain/(loss) |
| Change in unrealised gain/(loss) | |||
Equity price | |||||||||
Warrants | 94,959,244 | - | (3,171) | (14,034,141) | |||||
Equity swaps | 22,113,533 | 4,875,333 | 4,196,616 | 4,511,140 | |||||
Contingent value rights | 1,697,725 | - | - | 94,351 | |||||
118,770,502 |
| 4,875,333 |
| 4,193,445 |
| (9,428,650) | |||
31 December 2024 (audited) | 30 June 2024 (unaudited) | |||||||||
Primary underlying risk | Derivative assets |
| Derivative liabilities | Realised gain/(loss) |
| Change in unrealised gain/(loss) | ||||
Equity price | ||||||||||
Warrants | 88,047,316 | - | (19,848) | 51,901,210 | ||||||
Equity swaps | 20,526,482 | 7,799,422 | 5,175,290 | 11,672,409 | ||||||
Contingent value rights | 1,603,374 | - | - | (190,167) | ||||||
110,177,172 |
| 7,799,422 |
| 5,155,442 |
| 63,383,452 | ||||
5. Securities lending agreements
The Group has entered into securities lending agreements with its prime brokers. From time to time, the prime brokers lend securities on the Group's behalf. As of 30 June 2025 and 31 December 2024, no securities were loaned and no collateral was received.
6. Offsetting assets and liabilities
The Group is required to disclose the impact of offsetting assets and liabilities represented in the unaudited interim consolidated statement of assets and liabilities to enable users of the unaudited interim consolidated financial statements to evaluate the effect or potential effect of netting arrangements on its financial position for recognised assets and liabilities. These recognised assets and liabilities are financial instruments and derivative instruments that are either subject to an enforceable master netting arrangement or similar agreement or meet the following right of setoff criteria: the amounts owed by the Group to another party are determinable, the Group has the right to offset the amounts owed with the amounts owed by the other party, the Group intends to offset and the Group's right of setoff is enforceable by law.
As of 30 June 2025 and 31 December 2024, the Group held financial instruments and derivative instruments that were eligible for offset in the unaudited interim consolidated statement of assets and liabilities and are subject to a master netting arrangement. The master netting arrangement allows the counterparty to net applicable collateral held on behalf of the Group against applicable liabilities or payment obligations of the Group to the counterparty. These arrangements also allow the counterparty to net any of its applicable liabilities or payment obligations they have to the Group against any collateral sent to the Group.
As discussed in Note 1, the Group has elected not to offset assets and liabilities in the unaudited interim consolidated statement of assets and liabilities. The following table presents the potential effect of netting arrangements for asset derivative contracts presented in the unaudited interim consolidated statement of assets and liabilities:
Description | Gross amounts of recognised assets | Gross amounts offset in the unaudited interim consolidated statement of assets and liabilities | Gross amounts of recognised assets | 30 June 2025 (unaudited) Gross amounts not offset in the unaudited interim consolidated statement of assets and liabilities | ||||||||
| Financial instruments(a) | Cash collateral received(b) |
| Net amount | ||||||||
Equity swaps | ||||||||||||
Cowen Financial Products, LLC |
9,179,525 | - |
9,179,525 |
(1,822,632) | - |
7,356,893 | ||||||
Morgan Stanley & Co. LLC | 6,687,430 | - | 6,687,430 | (2,196,362) | - | 4,491,068 | ||||||
Bank of America Merrill Lynch |
5,084,820 | - |
5,084,820 |
(1,002) | - | 5,083,818 | ||||||
Jefferies & Co. | 1,093,123 | - | 1,093,123 | (845,554) | - | 247,569 | ||||||
J.P. Morgan Securities, LLC | 68,635 | - | 68,635 | - | - | 68,635 | ||||||
22,113,533 | - | 22,113,533 |
| (4,865,550) |
| - |
| 17,247,983 | ||||
Description | Gross amounts of recognised assets | Gross amounts offset in the consolidated statement of assets and liabilities | Gross amounts of recognised assets | 31 December 2024 (audited) Gross amounts not offset in the consolidated statement of assets and liabilities |
| ||||||||
| Financial instruments(a) | Cash collateral received(b) |
| Net amount | |||||||||
Equity swaps |
| ||||||||||||
Cowen Financial Products, LLC |
11,004,397 | - |
11,004,397 |
(3,666,923) | - | 7,337,474 |
| ||||||
Morgan Stanley & Co. LLC | 5,639,240 | - | 5,639,240 | (2,056,637) | - | 3,582,603 |
| ||||||
Bank of America Merrill Lynch |
3,411,345 | - |
3,411,345 |
(49) | - | 3,411,296 |
| ||||||
Jefferies & Co. | 471,500 | - | 471,500 | (471,500) | - | - |
| ||||||
20,526,482 | - | 20,526,482 |
| (6,195,109) |
| - |
| 14,331,373 |
| ||||
(a) Amounts related to master netting agreements (e.g. ISDA), determined by the Group to be legally enforceable in the event of default and if certain other criteria are met in accordance with applicable offsetting accounting guidance but were not offset due to management's accounting policy election.
(b) Amounts related to master netting agreements and collateral agreements determined by the Group to be legally enforceable in the event of default, but certain other criteria are not met in accordance with applicable offsetting accounting guidance. The collateral amounts may exceed the related net amounts of financial assets and liabilities presented in the unaudited interim consolidated statement of assets and liabilities. If this is the case, the total amount reported is limited to the net amounts of financial assets and liabilities with that counterparty.
The following tables present the potential effect of netting arrangements for liability derivative contracts presented in the unaudited interim consolidated statement of assets and liabilities as of 30 June 2025 and audited consolidated statement of assets and liabilities 31 December 2024:
Description | Gross amounts of recognised liabilities | Gross amounts offset in the consolidated statement of assets and liabilities | Gross amounts of recognised liabilities | 30 June 2025 (unaudited) Gross amounts not offset in the unaudited interim consolidated statement of assets and liabilities | Net amount | |||||
| Financial instruments(a) | Cash collateral pledged(b) |
| |||||||
Equity swaps | ||||||||||
Morgan Stanley & Co. LLC | 2,196,362 | - | 2,196,362 | (2,196,362) | - | - | ||||
Cowen Financial Products, LLC |
1,822,632 | - |
1,822,632 |
(1,822,632) | - | - | ||||
Jefferies & Co. | 845,554 | - | 845,554 | (845,554) | - | - | ||||
Goldman Sachs | 9,783 | - | 9,783 | - | - | 9,783 | ||||
Bank of America Merrill Lynch |
1,002 | - |
1,002 |
(1,002) | - | - | ||||
4,875,333 | - | 4,875,333 |
| (4,865,550) |
| - |
| 9,783 | ||
Description | Gross amounts of recognised liabilities | Gross amounts offset in the consolidated statement of assets and liabilities | Gross amounts of recognised liabilities | 31 December 2024 Gross amounts not offset in the consolidated statement of assets and liabilities | Net amount | |||||
| Financial instruments(a) | Cash collateral pledged(b) |
| |||||||
Equity swaps | ||||||||||
Cowen Financial Products, LLC |
3,666,923 | - |
3,666,923 |
(3,666,923) | - | - | ||||
Jefferies & Co. | 2,069,804 | - | 2,069,804 | (471,500) | (1,598,304) | - | ||||
Morgan Stanley & Co. LLC | 2,056,637 | - | 2,056,637 | (2,056,637) | - | - | ||||
J.P. Morgan Securities, LLC |
6,009 | - |
6,009 |
- | - | 6,009 | ||||
Bank of America Merrill Lynch |
49 | - |
49 |
(49) | - | - | ||||
7,799,422 | - | 7,799,422 |
| (6,195,109) |
| (1,598,304) |
| 6,009 | ||
(a) Amounts related to master netting agreements (e.g. ISDA), determined by the Group to be legally enforceable in the event of default and if certain other criteria are met in accordance with applicable offsetting accounting guidance but were not offset due to management's accounting policy election.
(b) Amounts related to master netting agreements and collateral agreements determined by the Group to be legally enforceable in the event of default, but certain other criteria are not met in accordance with applicable offsetting accounting guidance. The collateral amounts may exceed the related net amounts of financial assets and liabilities presented in the unaudited interim consolidated statement of assets and liabilities. If this is the case, the total amount reported is limited to the net amounts of financial assets and liabilities with that counterparty.
7. Securities sold short
The Group is subject to certain inherent risks arising from its investing activities of selling securities short. The ultimate cost to the Group to acquire these securities may exceed the liability reflected in these unaudited interim consolidated financial statements.
8. Risk factors
Some underlying investments may be deemed to be highly speculative investments and are not intended as a complete investment program. The Company is designed only for sophisticated persons who are able to bear the economic risk of the loss of their entire investment in the Company and who have a limited need for liquidity in their investment. The following risks are applicable to the Company:
Market risk
Certain events particular to each market in which Portfolio Companies conduct operations, as well as general economic and political conditions, may have a significant negative impact on the operations and profitability of the Group's investments and/or on the fair value of the Group's investments. Such events are beyond the Group's control, and the likelihood they may occur and the effect on the Group cannot be predicted. The Group intends to mitigate market risk generally by investing in Medtech and Biotech Companies in various geographies.
Portfolio Company products are subject to regulatory approvals and actions with new drugs, medical devices and procedures being subject to extensive regulatory scrutiny before approval, and approvals can be revoked.
The market value of the Group's holdings in public Portfolio Companies could be affected by a number of factors, including, but not limited to: a change in sentiment in the market regarding the public Portfolio Companies, the market's appetite for specific asset classes; and the financial or operational performance of the public Portfolio Companies.
The size of investments in public Portfolio Companies or involvement in management may trigger restrictions on buying or selling securities. Laws and regulations relating to takeovers and inside information may restrict the ability of the Group to carry out transactions, or there may be delays or disclosure requirements before transactions can be completed.
Equity prices and returns from investing in equity markets are sensitive to various factors, including but not limited to: expectations of future dividends and profits; economic growth; exchange rates; interest rates; and inflation.
Biotech/healthcare companies
The Portfolio Companies are biotechnology and medical technology companies, which are generally subject to greater governmental regulation than other industries at both the state and federal levels. Changes in governmental policies may have a material effect on the demand for or costs of certain products and services.
Any failure by a Portfolio Company to develop new technologies or to accurately evaluate the technical or commercial prospects of new technologies could result in it failing to achieve a growth in value and this could have a material adverse effect on the Group's financial condition.
Portfolio Companies may not successfully translate promising scientific theory into a commercially viable business opportunity. Further, the Portfolio Companies' therapies in development may fail clinical trials and therefore no longer be viable.
Portfolio Company products are subject to intense competition and there are many factors that will affect whether the new therapies released by the Portfolio Companies gain market share against competitors and existing therapies.
Portfolio Companies may be newer small and mid-size Medtech and Biotech Companies. These companies may be more volatile and have less experience and fewer resources than more established companies.
Concentration risk
The Group may not make an investment or a series of investments in a Portfolio Company that result in the Group's aggregate investment in such Portfolio Company exceeding 15 per cent. of the Group's gross assets, save for Rocket for which the limit is 25 per cent. as stated in the Company's Prospectus. Each of these investment restrictions will be calculated as at the time of investment. As such, it is possible that the Group's portfolio may be concentrated at any given point in time, potentially with more than 15 per cent. of gross assets held in one Portfolio Company as Portfolio Companies increase or decrease in value following such initial investment. The Group's portfolio of investments may also lack diversification among Medtech and Biotech Companies and related investments.
Concentration of credit risk
In the normal course of business, the Group maintains its cash balances in financial institutions, which at times may exceed US federal, Guernsey or UK insured limits, as applicable. The Group is subject to credit risk to the extent any financial institution with which it conducts business is unable to fulfil contractual obligations on its behalf. Management monitors the financial condition of such financial institutions and does not anticipate any losses from these counterparties.
Counterparty risk
The Group invests in equity swaps and takes the risk of non-performance by the other party to the contract. This risk may include credit risk of the counterparty, the risk of settlement default, and generally, the risk of the inability of counterparties to perform with respect to transactions, whether due to insolvency, bankruptcy or other causes.
In an effort to mitigate such risks, the Group will attempt to limit its transactions to counterparties which are established, well capitalised and creditworthy.
Liquidity risk
Liquidity risk is the risk that the Group cannot meet its financial commitments as they fall due. The Group's unquoted investments may have limited or no secondary market liquidity so the Investment Manager maintains a sufficient balance of cash and market quoted securities which can be sold if needed to meet its commitments.
The Group's investments in quoted securities may also be subject to sale restrictions on listing and when the Investment Manager is subject to close periods or privy to confidential information by virtue of their active involvement in the management of portfolio companies.
Derivative transactions may not be liquid in all circumstances, such that in volatile markets it may not be possible to close out a position without incurring a loss. The illiquidity of the derivatives markets may be due to various factors, including congestion, disorderly markets, limitations on deliverable supplies, the participation of speculators, government regulation and intervention, and technical and operational or system failures.
Foreign exchange risk
The Group will make investments in various jurisdictions in a number of currencies and will be exposed to the risk of currency fluctuations that may materially adversely affect, amongst other things, the value of the Portfolio Company or the Group's investment in such Portfolio Company, or any distributions received from the Portfolio Company. Under its investment policy, the Group does not intend to enter into any securities or financially engineered products designed to hedge portfolio exposure or mitigate portfolio risk as a core part of its investment strategy.
9. Share capital
During the period ended 30 June 2025, the Company share activity was as follows:
| 30 June 2025 (unaudited) | 30 June 2025 (unaudited) | 31 December 2024 (audited) | 31 December 2024 (audited) | |
|
Number of Ordinary Shares |
Number of Treasury Shares | Number of Ordinary Shares | Number of Treasury Shares | |
As at 1 January | 335,713,649 | 10,253,791 | 210,635,347 | 1,753,791 | |
Share issuance | - | - | 133,578,302 | - | |
Share buyback | (5,650,000) | 5,650,000 | (8,500,000) | 8,500,000 | |
As at 30 June/31 December | 330,063,649 | 15,903,791 | 335,713,649 | 10,253,791 | |
During the period ended 30 June 2025, the Company bought back 5,650,000 (31 December 2024: 8,500,000) Ordinary Shares at an average price of $1.19 (31 December 2024: $1.33) for a total cost of $6,708,388 (31 December 2024: $11,340,306), including transaction costs of $10,147 (31 December 2024: $22,861). At the date of approval of these consolidated financial statements, all 15,903,791 of repurchased Ordinary Shares were held as treasury shares (31 December 2024: 10,253,791).
During the year ended 31 December 2024, the Company issued 181,901,165 new shares to facilitate the acquisition of Arix Bioscience plc in an all‐share transaction for $246,476,079 with associated issuance costs of $6,473,897. Of the 181,901,165 new shares, 48,322,863, with a value of $59,221,117, were issued to the Group as existing shareholders of Arix Bioscience plc, and were subsequently cancelled. The details around this transaction are further disclosed within the unaudited interim consolidated statement of cash flows and within Note 1. No new shares were issued during the period ended 30 June 2025.
Ordinary Shares carry the right to receive all income of the Company attributable to the Ordinary Shares and to participate in any distribution of such income made by the Company. Such income shall be divided pari passu among the holders of Ordinary Shares in proportion to the number of Ordinary Shares held by them.
Ordinary Shares shall carry the right to receive notice of and attend and vote at any general meeting of the Company, and at any such meeting on a show of hands, every holder of Ordinary Shares present in person (includes present by attorney or by proxy or, in the case of a corporate member, by duly authorised corporate representative) and entitled to vote shall have one vote, and on a poll, subject to any special voting powers or restrictions, every holder of Ordinary Shares present in person or by proxy shall be entitled to one vote for each Ordinary Share, or fraction of an Ordinary Share, held.
On 1 December 2022, the Performance Allocation Share held by RTW Venture Performance LLC was surrendered in exchange for a New Performance Allocation Share issued by the Subsidiary. The New Performance Allocation Share issued by the Subsidiary has identical terms to the original Performance Allocation Share issued by the Company. From 1 December 2022, the Performance Allocation Amount is now allocated at the Subsidiary level, and is presented in the Group's financial statements as part of the Non-Controlling Interest. The sole New Performance Allocation Share is held by RTW Venture Performance LLC. As at 30 June 2025, there were no Performance Allocation Shares of the Company in issue (31 December 2024: nil) and one New Performance Allocation Share of the Subsidiary in issue (31 December 2024: one).
New Performance Allocation Shares of the Subsidiary carry the right to receive, and participate in, any dividends or other distributions of the Subsidiary available for dividend or distribution. New Performance Allocation Shares are not entitled to receive notice of, to attend or to vote at general meetings of the Company or the Subsidiary.
For all share classes, subject to compliance with the solvency test set out in the Companies Law, the Board may declare and pay such annual or interim dividends and distributions as appear to be justified by the position of the Group. The Board may, in relation to any dividend or distribution, direct that the dividend or distribution shall be satisfied wholly or partly by the distribution of assets, and in particular of paid-up shares or reserves of any nature as approved by the Group.
10. Related party transactions
Management Fee
The Investment Manager receives a monthly management fee, in advance, as of the beginning of each month in an amount equal to 0.104% (1.25% per annum) of the net assets of the Group (the "Management Fee"). For purposes of determining the Management Fee, private investments will be valued at the fair value. The Management Fee will be prorated for any period that is less than a full month.
The Management Fees charged for the period ended 30 June 2025 amounted to $3,668,870 (period ended 30 June 2024: $3,436,352) of which $nil (31 December 2024: $nil) was outstanding at the period end.
Performance Allocation
The Performance Allocation Share held by RTW Venture Performance LLC was surrendered in exchange for a New Performance Allocation Share issued by the Subsidiary. The New Performance Allocation Share issued by the Subsidiary has identical terms to the original Performance Allocation Share issued by the Company.
In respect of each Performance Allocation Period, the Performance Allocation Amount shall be allocated at the Subsidiary level and disclosed on the Group's financial statements within the Non-Controlling Interest, subject to the satisfaction of a hurdle condition.
The Performance Allocation Amount relating to the Performance Allocation Period, which is calculated solely at the Subsidiary, is an amount equal to:
((A-B) x C) x 20 per cent.
where:
A is the Adjusted Net Asset Value per Ordinary Share on the Calculation Date, adjusted by:
adding back (i) the total net Distributions (if any) per Ordinary Share (whether paid, or declared but not yet paid) during the Performance Allocation Period; and (ii) any accrual for the Performance Allocation for the current Performance Allocation Period reflected in the Net Asset Value per Ordinary Share; and deducting any accretion in the Net Asset Value per Ordinary Share resulting from either the issuance of Ordinary Shares at a premium or the repurchase or redemption of Ordinary Shares at a discount during the Performance Allocation Period;
B is the Adjusted Net Asset Value per Ordinary Share at the start of the Performance Allocation Period; and
C is the time weighted average number of Ordinary Shares in issue during the Performance Allocation Period.
The Hurdle Amount represents an 8 per cent. annualised compounded rate of return in respect of the Adjusted Net Asset Value per Ordinary Share from the start of the initial Performance Allocation Period through the then current Performance Allocation Period.
The Performance Allocation Share Class can elect to receive the Performance Allocation Amount in Ordinary Shares, cash, or a mixture of the two, subject to a minimum 50% as Ordinary Shares. The Performance Allocation Share Class entered into a letter agreement dated 21 April 2020, pursuant to which the Performance Allocation Share Class agreed to defer distributions of Ordinary Shares that would otherwise be distributed to the Performance Allocation Share Class no later than 30 business days after the publication of the Group's audited annual consolidated financial statements. Under that letter agreement, such Ordinary Shares shall be distributed to the Performance Allocation Share Class at such time or times as determined by the Boards of Directors of the Group.
The Group will increase or decrease the amount owed to the Performance Allocation Share Class based on its investment exposure to the Group's performance had such Performance Ordinary Shares been so issued. The Performance Allocation Amount for the period ended 30 June 2024 includes the residual, undistributed Performance Allocation Amounts from prior years that were previously converted into a total of 14,228,208 Notional Ordinary Shares.
These Notional Ordinary Shares are subject to market risk alongside the Ordinary Shares and incurred a mark to market loss of $1,538,582 in 2025 (31 December 2024: mark to market loss of $1,259,780), which is included in Performance Allocation within the unaudited interim consolidated statement of changes in net assets. There was no reallocation of uncrystallized performance allocation from Ordinary Shareholders to the Performance Allocation Share Class related to the Group's performance in the period (31 December 2024: reversal of $2,756,842).
Until the Group makes a distribution of Ordinary Shares to the Performance Allocation Share Class, the Group will have an unsecured discretionary obligation to make such distribution at such time or times as the Board of Directors of the Group determines. RTW Venture Performance LLC has agreed to the deferral of the distributions of the Subsidiary's Ordinary Shares in connection with its own tax planning. The Group does not believe that the deferral of such distributions to the Performance Allocation Share Class will have any negative effects on holders of Ordinary Shares.
RTW Venture Performance LLC, an affiliate of the Investment Manager is a member of the Performance Allocation Share Class and will therefore receive a proportion of the Performance Allocation Amount. For the period ended 30 June 2025, the Board did not approve a cash distribution to the Performance Allocation Share Class (period ended 30 June 2024: $nil). At the period end, the Performance Allocation Share Class of the Subsidiary is reflected within the Non-Controlling Interest balance of $24,183,942 (31 December 2024: $25,722,524).
Other related party transactions
The Investment Manager is also refunded any research costs incurred on behalf of the Group.
On 6 July 2023, the Group signed a capital commitment to 4010 Royalty Fund, a private fund created and managed by RTW Investments, LP. At 30 June 2025, $21,057,168 of the Group's $31,485,000 capital commitment remained unfunded (31 December 2024: $14,651,294 of $25,000,000). No management or performance fees are charged to the Group at the 4010 Royalty Fund.
Director fees and interests
One of the Directors of the Group, Stephanie Sirota, is also a partner and the Chief Business Officer of the Investment Manager.
As at 30 June 2025, the number of Ordinary Shares held by each Director was as follows:
| 30 June 2025 (unaudited) | 31 December 2024 (audited) | ||
|
Number of Ordinary Shares |
Number of Ordinary Shares | ||
William Simpson | 255,000 | 200,000 | ||
Paul Le Page | 178,000 | 128,000 | ||
William Scott | 400,000 | 400,000 | ||
Nicola Blackwood | - | - | ||
Stephanie Sirota | 1,010,000 | 1,010,000 |
Roderick Wong is a major shareholder and a member of the Investment Manager. Roderick Wong serves on the boards of the following investments: Rocket, Corxel Pharmaceuticals, HSA2 Holdings, LLC and Yarrow Biotechnology. As at 30 June 2025, he held 50,356,880 Ordinary Shares in the Group (15.26% of the Ordinary Shares in issue) (31 December 2024: 49,643,313, 14.79% of the Ordinary Shares in issue).
The total Directors' fees expense for the period amounted to $151,927 (30 June 2024: $117,976) of which $80,101 was outstanding at 30 June 2025 (31 December 2024: $71,029) and is included within accrued expenses.
All of the Directors of the Company are also directors of the Subsidiary. Each has served since the Subsidiary's incorporation on 23 November 2022, except Baroness Blackwood, who was appointed a director of the Subsidiary alongside her appointment as director of the Company on 11 July 2024. Stephanie Sirota is also a director of the UK Subsidiary.
Incubated Companies
The Group invests in RTW incubated companies. Incubated companies are those portfolio companies that are formed and supported by RTW ("Incubated Companies"). Incubated Companies generally are small, emerging companies that are unseasoned, unprofitable and/or have no established operating history or earnings. These companies may also lack technical, marketing, financial and other resources or may be dependent upon the success of one product or service or the effectiveness of RTW and its management team.
Employees of RTW and employees of certain RTW affiliates are expected to serve as executives, officers, directors, members, consultants or employees of such companies. These individuals are eligible for compensation in the Incubated Companies in the form of founder shares or other forms of company securities. Certain RTW employees who perform specific executive functions for such Incubated Companies may also receive cash compensation directly or indirectly from those companies. For the avoidance of doubt, these employees do not receive such compensation from both RTW and the Incubated Company. These employees receive 100% of their compensation from RTW and RTW charges back to the Incubated Company for the applicable percentage of their time spent on executive functions at the Incubated Company. Employees of RTW and employees of certain RTW affiliates may also receive compensation in the form of stock options or other securities from certain Incubated Companies in connection with their delivery of specified products, research and consulting services. RTW believes this is an effective way to align incentives and motivate employees, while reducing the financial burden on the newly Incubated Companies by minimizing the need to hire external employees.
During the period ended 30 June 2025, the Group entered into a purchase transaction with affiliated entities also managed by the Investment Manager. A total purchase of $6,900,000 was made at fair value with these related parties.
11. Administrative services
Altum (Guernsey) Limited (previously named Elysium Fund Management Limited ("Altum")) serves as Administrator to the Group, providing administration, corporate secretarial, corporate governance and compliance services. Morgan Stanley Fund Services USA LLC ("MSFS") serves as the Group's Sub-Administrator.
During the period ended 30 June 2025, Altum and MSFS charged administration fees of $197,160 and $177,625 respectively (period ended 30 June 2024: Altum charged $185,359 and MSFS charged $171,337), of which a prepayment of $7,605 and an accrual of $97,079 (31 December 2024: Altum prepayment of $5,693, MSFS accrual of $105,860) were outstanding at 30 June 2025, and were included within accrued expenses.
12. Financial highlights
Financial highlights for the six month period ended 30 June 2025, six month period ended 30 June 2024 and year ended 31 December 2024 are as follows:
| 30 June 2025 (unaudited) | 30 June 2024 (unaudited) | 31 December 2024 (audited) | ||||
Per Ordinary Share operating performance | |||||||
Net Asset Value, beginning of period/year | $ 1.81 | $ 1.90 | $ 1.90 | ||||
Cost of issuance of Ordinary Shares | - | (0.23) | (0.23) | ||||
Share buybacks | 0.01 | 0.03 | 0.03 | ||||
Income from investments | |||||||
Net investment income/(loss) | 0.06 | (0.01) | (0.03) | ||||
Net realised and unrealised gain/(loss) on securities, derivatives and foreign currency transactions | (0.18) | 0.26 |
0.13 | ||||
Income/(loss) attributable to Non-Controlling Interest | 0.00 | 0.00 |
0.01 | ||||
Total from investment operations | (0.12) | 0.25 | 0.11 | ||||
Net Asset Value, end of period/year | $1.70 | $1.95 | $1.81 | ||||
|
| ||||||
Total return | |||||||
Total return before Performance Allocation | (5.98) % | 2.49 % | (5.25) % | ||||
Performance Allocation (excluding mark to market) | 0.00 % | 0.50 % | 0.62 % | ||||
Total return after Performance Allocation | (5.98)% | 2.99 % | (4.63) % | ||||
Ratios to average net assets* | |||||||
Expenses | 1.78 % | 1.28 % | 2.78 % | ||||
Performance Allocation (including mark to market) | (0.26) % | (0.15) % | (0.66) % | ||||
Expenses and Performance Allocation | 1.52 % | 1.13 % | 2.12 % | ||||
Net investment income/(loss) | 3.29 % | (0.76) % | (1.44) % | ||||
NAV total return for the period/year | (5.98) % | 2.99 % | (4.63) % | ||||
| |||||||
* Ratios are not annualised.
Financial highlights are calculated for Ordinary Shares. An individual shareholder's financial highlights may vary based on the timing of capital share transactions. Net investment income/loss does not reflect the effects of the Performance Allocation.
13. Subsequent events
These unaudited interim consolidated financial statements were approved by the Board of Directors on 10 September 2025. Subsequent events have been evaluated through this date.
General Company Information
Structure | Closed-end Investment Fund |
Domicile | Guernsey |
Listing | London Stock Exchange Main Market |
Launch date | 30 October 2019 |
Dividend policy | To be reinvested |
Management fee | 1.25% |
Performance fee | 20% with an 8.0%, annualised and compounded- since-inception, hurdle |
ISIN | GG00BKTRRM22 |
SEDOL | BKTRRM2 |
Ticker | RTW |
LEI | 549300Q7EXQQH6KF7Z84 |
Website | www.rtwbio.com |
Defined Terms
"Adjusted Net Asset Value" | the Net Asset Value adjusted by deducting the unrealised gains and unrealised losses in respect of private Portfolio Companies; |
"AIC" | the Association of Investment Companies; |
"AIC Code" | the AIC Code of Corporate Governance dated February 2019; |
"AIFMD" | the Alternative Investment Fund Managers Directive; |
"Antibody" | a large Y-shaped blood protein that can stick to the surface of a virus, bacteria, or receptor on a cell; |
"Antibody-Oligonucleotide Conjugates" or "AOC" | molecules that combine structures of an antibody and an oligo; |
"Arix" | Arix Biosciences plc, the company whose assets the Company acquired in February 2024; |
"ASCO" | American Society of Clinical Oncology, also the name of the Society's annual oncology conference; |
"Autoimmune diseases" | conditions, where the immune system mistakenly attacks a body tissue; |
"BLA" | Biologics License Application, a request for permission to introduce, or deliver for introduction, a biologic product into interstate commerce (in the U.S.): |
"Calculation Date" | 30 June or, if such date is not a business day, the previous business day; |
"Cardiovascular disease" | conditions affecting heart and vascular system; |
"CNS" | Central Nervous System; |
"Companies Law" | the Companies (Guernsey) Law, 2008 (as amended); |
"the Company" | RTW Biotech Opportunities Ltd (or RTW Bio) is a company incorporated in Guernsey as a closed-ended Investment Company; |
"CRS" | Common Reporting Standard; |
"Danon Disease" | a rare genetic heart condition in children, predominantly boys; |
"DTR" | Disclosure Guidance and Transparency Rules of the UK's FCA; |
"Fanconi Anaemia" | a rare genetic blood condition in young children; |
"FATCA" | the Foreign Account Tax Compliance Act; |
"FCA" | the Financial Conduct Authority; |
"FDA" | the United States Food and Drug Administration; |
"FRC" | the Financial Reporting Council; |
"FTC" | the Federal Trade Commission; |
"Gene therapy" | a biotechnology that uses gene delivery systems to treat or prevent a disease; |
"Genetic Medicine" | an approach to treat or prevent a disease using gene therapy or RNA medicines; |
"GFSC" | the Guernsey Financial Services Commission; |
"GFSC Code"
| the GFSC Finance Sector Code of Corporate Governance as amended in June 2021; |
"Greater China" | encompasses mainland China, Macau, Hong Kong and Taiwan; |
"the Group" | the Company and its subsidiaries, RTW Biotech Opportunities Operating Ltd and RTW Biotech UK Ltd; |
"HCM" or "Hypertrophic cardiomyopathy" | a cardiovascular disease characterised by an abnormally thick heart muscle; |
"Investigational New Drug" or "IND" | the FDA's investigational New Drug program is the means by which a pharmaceutical company obtains permission to start human clinical trials; |
"Investment Manager" | RTW Investments, LP, also called RTW Investments or RTW; |
"IPEV"
| the International Private Equity and Venture Capital Valuation (IPEV) Guidelines set out recommendations, intended to represent current best practice, on the valuation of Private Capital Investments; |
"IRA" | Inflation Reduction Act of 2022; |
"Lentiviral vector or "LVV" | based gene therapy - a type of viral vector used to deliver a gene; |
"Leukocyte adhesion deficiency" or "LAD-I" | a rare genetic disorder of immunodeficiency in young children; |
"Multi-omics" | a biological analysis approach in which the data sets are multiple "omes", such as the genome, proteome, transcriptome, epigenome, metabolome, and microbiome; |
"Myotonic Dystrophy" | a genetic condition that affects muscle function; |
"Nasdaq Biotech" or "NBI" | a stock market index made up of securities of NASDAQ-listed companies classified according to the Industry Classification Benchmark as either the Biotechnology or the Pharmaceutical industry; |
"New Performance Allocation Shares" | performance allocation shares of no-par value in the capital of the Subsidiary; |
"NewCo" | a company conceived and incubated by RTW Investments, LP; |
"Notional Ordinary Shares" | Performance Ordinary Shares, in which receipt of such shares has been deferred; |
"Oligonucleotides" or "Oligos" | short DNA or RNA molecules that have a wide range of applications in genetic testing and research; |
"Omics" | any of several areas of biological study defined by the investigation of the entire complement of a specific type of biomolecule or the totality of a molecular process within an organism. In biology the word omics refers to the sum of constituents within a cell. The omics sciences share the overarching aim of identifying, describing, and quantifying the biomolecules and molecular processes that contribute to the form and function of cells and tissues; |
"Oncology" | a therapeutic area focused on diagnosis, prevention, and treatment of cancer; |
"Performance Allocation Shares" | performance allocation shares of no-par value in the capital of the Company (prior to the 1 December 2022 reorganisation), or performance allocation shares of no-par value in the capital of the Subsidiary (with effect from the 1 December 2022 reorganisation); |
"Performance Allocation Period" | each period ending on a Calculation Date and beginning on the business day immediately following the last Performance Allocation Period in respect of which a Performance Allocation has been allocated; |
"PRAME" | a cancer-testis antigen (CTA) that is highly expressed in a broad range of solid and hematologic malignancies; |
"Pyruvate Kinase Deficiency" or "PKD" | a rare genetic disorder affecting red blood cells; |
"Radiopharmaceuticals" | pharmaceutical consisting of a radioactive compound used in radiation therapy; |
"RTW" | RTW Investments, LP, also referred to as the Investment Manager; |
"Russell 2000 Biotechnology Index" or "RGUSHSBT" | a stock index of small cap biotechnology and pharmaceutical companies; |
"Small molecule" | a compound that can regulate a biologic activity; |
"SPV" | RTW Biotech ALI LLC; |
"the Subsidiary" or "OpCo" | RTW Biotech Opportunities Operating Ltd; |
"the UK Subsidiary" | RTW Biotech UK Limited; |
"Tachycardia" | a heart rhythm disorder; |
"UK AIFMD"
| refers to a domestic regime of laws regulating the management and marketing of alternative investment funds and fund managers in the UK, which generally maintains the rules set out in the European Union's AIFMD as implemented at the end of the transition period following Brexit; |
"UK Code" | the UK Corporate Governance Code 2018 published by the Financial Reporting Council in July 2018; |
"UK-Guernsey IGA" | The UK-Guernsey Intergovernmental Agreement for the Automatic Exchange of Information; |
"Ulcerative Colitis" | an inflammatory bowel disease that causes sores in the digestive tract; |
"Uveal melanoma" | a type of eye cancer; |
"WACC" | weighted average cost of capital; |
"XBI" | the SPDR S&P Biotech ETF; |
"XIRR" | an internal rate of return calculated using irregular time intervals. |
Listing of portfolio company abbreviations used throughout this report
Shorthand Company Name | Legal Company Name |
Acadia | Acadia Pharmaceuticals, Inc. |
Akero | Akero Therapeutics, Inc. |
ALI | American Laboratories, Inc. |
Apogee | Apogee Therapeutics, Inc. |
Argenx | argenx SE |
Artios | Artios Pharma, Inc. |
Avidity | Avidity Biosciences, Inc. |
Beta Bionics | Beta Bionics, Inc. |
Compass | Compass Pathways plc |
Corxel | Corxel Pharmaceuticals |
Dyne | Dyne Therapeutics, Inc. |
Ensoma | Ensoma, Inc. |
Evommune | Evommune, Inc. |
GH Research | GH Research PLC |
Jade | Jade Biosciences |
Kailera | Kailera Therapeutics, Inc. |
Lycia | Lycia Therapeutics, Inc. |
Madrigal | Madrigal Pharmaceuticals, Inc. |
Merus | Merus N.V. |
Milestone | Milestone Pharmaceuticals, Inc. |
Prolium | Prolium Bioscience, Inc. |
Protagonist | Protagonist Therapeutics, Inc. |
PTC | PTC Therapeutics, Inc. |
Rocket | Rocket Pharmaceuticals, Inc. |
RTW Royalty 2 | RTW Fund 2 (royalty deal for Jelmyto) |
RTW Royalty Fund | 4010 Royalty Fund |
Stoke | Stoke Therapeutics, Inc. |
Tarsus | Tarsus, Pharmaceuticals, Inc. |
Taysha | Taysha Gene Therapies, Inc. |
UniQure | uniQure biopharma B.V. |
UroGen | UroGen Pharma |
Verastem | Verastem, Inc. |
Verona | Verona Pharma plc |
Zai Lab | Zai Lab Limited |
Alternative Performance Measures unaudited
APM | Definition | Purpose | Calculation |
Available Cash | Cash held by the Group's Bankers, Prime Broker and an ISDA counterparty. | A measure of the Group's liquidity, working capital and investment level. | Cash and cash equivalents, Due from brokers, Receivable from unsettled trades and other miscellaneous current assets, less Due to brokers, Payable for unsettled trades and other miscellaneous current liabilities on the Statement of Assets & Liabilities. |
NAV per Ordinary Share | The Group's NAV divided by the number of Ordinary Shares. | A measure of the value of one Ordinary Share. | The net assets attributable to Ordinary Shares on the statement of financial position divided by the number of Ordinary Shares in issue as at the calculation date. |
Price per share | The Company's closing share price on the London Stock Exchange for a specified date. | A measure of the supply and demand for the Company's shares. | Extracted from the official list of the London Stock Exchange. |
NAV Growth | The percentage increase/decrease in the NAV per Ordinary share during the reporting period. | A key measure of the success of the Investment Manager's investment strategy. | The quotient of the NAV per share at the end of the period and the NAV per share at the beginning of the period minus one expressed as a percentage. |
Share price growth/Total Shareholder Return | The percentage increase(decrease) in the price per share during the reporting period. | A measure of the return that could have been obtained by holding a share over the reporting period. | The quotient of the price per share at the end of the period and the price per share at the beginning of the period minus one expressed as a percentage. The measure excludes transaction costs. |
Share Price Premium/ (Discount) | The amount by which the Ordinary Share price is higher/lower than the NAV per Ordinary Share, expressed as a percentage of the NAV per ordinary share. | A key measure of supply and demand for the Company's shares. A premium implies excess demand versus supply and vice versa. | The quotient of the price per share at the end of the period and the NAV per share at the end of the period minus one expressed as a percentage. |
Multiple on Invested Capital (MOIC or MOC) | The multiple that measures value that an investment has generated. | A measure to evaluate performance of the realised and unrealised investments. | The ratio between initial capital invested in a portfolio company and current value of the investment. It is a gross metric and calculation is performed before fees and incentive. |
Extended Internal Rate of Return (XIRR) | The percentage or single rate of return when applied to all transactions in a portfolio company. | A measure of return which is used when multiple investments have been made over time into a portfolio company. | The rate also expressed as a percentage that calculates the returns on the total investment made with increments through a given period (from initial investment date to 30 June 2023). |
Ongoing Charges Ratio | The recurring costs that the Group has incurred during the period excluding performance fees and one-off legal and professional fees, expressed as a percentage of the Group's average NAV for the period. | A measure of the minimum gross profit that the Group needs to produce to make a positive return for shareholders. | Calculated in accordance with the AIC methodology detailed at the web link below: https://www.theaic.co.uk/sites/default/files/documents/AICOngoingChargesCalculationMay12.pdf
|
Leverage | As defined by the AIFMD, any method by which the AIFM increases the exposure of an AIF it manages, whether through borrowing of cash securities, or leverage embedded in derivative positions or by any other means. | A measure of the excess of the Group's investments exposure over its total net assets. | Calculated in accordance with the AIFMD's gross and commitment methodologies as outlined in Articles 7 and 8 of the Delegated Regulation 231/2013: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013R0231 |
Schedule of Key Service Providers
Board of Directors Mr William Simpson (Chair, Chair of Management Engagement Committee, Chair of Sustainability Committee) Mr Paul Le Page (Chair of Audit Committee) Mr William Scott (Chair of Nomination & Remuneration Committee) Baroness Nicola Blackwood (Senior Independent Non-Executive Director) Ms Stephanie Sirota
Investment Manager and AIFM RTW Investments, LP 40 10th Avenue New York NY 10014 USA
Registered Office 1st Floor, Royal Chambers St Julian's Avenue St Peter Port Guernsey GY1 3JX
Administrator and Company Secretary Altum (Guernsey) Limited 1st Floor, Royal Chambers St Julian's Avenue St Peter Port Guernsey GY1 3JX
Sub-Administrator Morgan Stanley Fund Services USA LLC 2000 Westchester Avenue, 1st Floor Purchase NY 10577 United States of America
| Guernsey Advocates Carey Olsen (Guernsey) LLP Carey House Les Banques St Peter Port Guernsey GY1 4BZ
UK Legal Advisers Herbert Smith Freehills Kramer LLP Exchange House Primrose Street London EC2A 2EG
Corporate Brokers BofA Securities 2 King Edward Street London EC1A 1HQ
Deutsche Numis Securities 45 Gresham Street London EC2V 7BF
Registrar MUFG Corporate Markets (Guernsey) Limited Mont Crevelt House Bulwer Avenue St Sampson Guernsey GY2 4LH
Sustainability Consultant Terra Instinct 40-41 Pall Mall London SW1Y 5JQ | Distribution/IR and Public Relations Partner Cadarn Capital c/o WeWork 1 Fore Street Avenue London EC2Y 9DT
Auditor KPMG Channel Islands Limited Glategny Court Glategny Esplanade St Peter Port Guernsey GY1 1WR
Principal Bankers Barclays Bank PLC, Guernsey Branch Le Marchant House Le Truchot St Peter Port Guernsey GY1 3BE
|
Related Shares:
Rtw Biotech